Flask Contains 4.4 G Of . That means 1 mole of carbon dioxide = 44. We know, molar mass of carbon dioxide = 44 grams. Calculate the molar mass of co2: 44 g ( molecular mass) of co2 = 1 mole of co2. A flask contains 4.4g of c o 2 gas. X = 0.1 mole of co2 gas. The mass in grams is 6.02 with 10 being. Calculate the molar mass of co2: (b)how many molecules of c o 2 gas are. Let's take a look at this question. 4.4 g of co2 = x mole of co2. Calculate (b) how many moles of c o 2 gas does it contain? 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by avogadro's number to get. Now, calculate the number of moles of co2 you have: C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) = 44.01 g/mol.
from www.numerade.com
Calculate (b) how many moles of c o 2 gas does it contain? A flask contains 4.4g of c o 2 gas. We need to find the number of co2 molecule in 44 grams. Calculate the molar mass of co2: X = 0.1 mole of co2 gas. The mass in grams is 6.02 with 10 being. C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) = 44.01 g/mol. 44 g ( molecular mass) of co2 = 1 mole of co2. Let's take a look at this question. Now, calculate the number of moles of co2 you have:
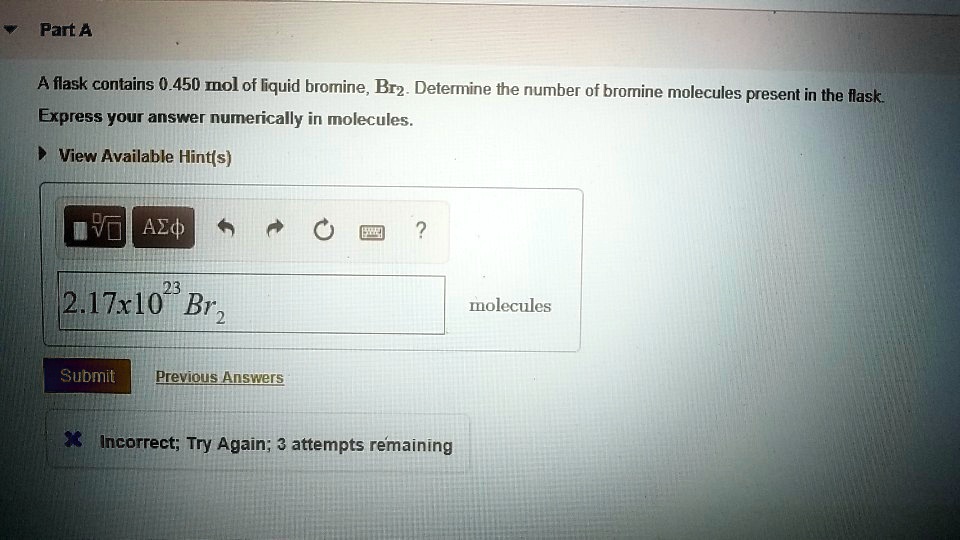
SOLVED Part A A flask contains 0.450 mol of liquid bromine, Br2
Flask Contains 4.4 G Of Let's take a look at this question. Calculate the molar mass of co2: 4.4 g of co2 = x mole of co2. We know, molar mass of carbon dioxide = 44 grams. 44 g ( molecular mass) of co2 = 1 mole of co2. X = 0.1 mole of co2 gas. The mass in grams is 6.02 with 10 being. Calculate the molar mass of co2: A flask contains 4.4g of c o 2 gas. 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by avogadro's number to get. Now, calculate the number of moles of co2 you have: 8 people found it helpful. (b)how many molecules of c o 2 gas are. C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) = 44.01 g/mol. Calculate (b) how many moles of c o 2 gas does it contain? Let's take a look at this question.
From www.chegg.com
Solved The flask shown here contains 10.0 mL of HCI and a Flask Contains 4.4 G Of That means 1 mole of carbon dioxide = 44. 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by avogadro's number to get. 44 g ( molecular mass) of co2 = 1 mole of co2. Calculate the molar mass of co2: The mass in grams is 6.02 with 10 being. Calculate (b) how many moles of c o 2. Flask Contains 4.4 G Of.
From www.chegg.com
Solved Which flask below. A or B. represents an accurate Flask Contains 4.4 G Of Calculate (b) how many moles of c o 2 gas does it contain? 8 people found it helpful. A flask contains 4.4g of c o 2 gas. Now, calculate the number of moles of co2 you have: Let's take a look at this question. Calculate the molar mass of co2: X = 0.1 mole of co2 gas. We know, molar. Flask Contains 4.4 G Of.
From www.labsupply.co.nz
Flasks Flask, Cell Culture, 250mL, PS, clear, TC treated, Sterile, D Flask Contains 4.4 G Of 44 g ( molecular mass) of co2 = 1 mole of co2. C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) = 44.01 g/mol. Calculate the molar mass of co2: Let's take a look at this question. Now, calculate the number of moles of co2 you have: (b)how many molecules of. Flask Contains 4.4 G Of.
From www.alamy.com
Conical flask cutout hires stock photography and images Alamy Flask Contains 4.4 G Of Calculate the molar mass of co2: The mass in grams is 6.02 with 10 being. 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by avogadro's number to get. 8 people found it helpful. That means 1 mole of carbon dioxide = 44. Calculate (b) how many moles of c o 2 gas does it contain? Let's take a. Flask Contains 4.4 G Of.
From www.chegg.com
Solved A sealed flask contains the following substances 1.25 Flask Contains 4.4 G Of X = 0.1 mole of co2 gas. The mass in grams is 6.02 with 10 being. Now, calculate the number of moles of co2 you have: Calculate the molar mass of co2: Calculate (b) how many moles of c o 2 gas does it contain? We need to find the number of co2 molecule in 44 grams. C = 12.01. Flask Contains 4.4 G Of.
From www.numerade.com
SOLVED Part A A flask contains 0.450 mol of liquid bromine, Br2 Flask Contains 4.4 G Of X = 0.1 mole of co2 gas. We need to find the number of co2 molecule in 44 grams. Calculate the molar mass of co2: Calculate (b) how many moles of c o 2 gas does it contain? 8 people found it helpful. The mass in grams is 6.02 with 10 being. Calculate the molar mass of co2: (b)how many. Flask Contains 4.4 G Of.
From www.indiamart.com
Conical JAISBO Bottle Neck Flask at Rs 190 in New Delhi ID 21017618891 Flask Contains 4.4 G Of We know, molar mass of carbon dioxide = 44 grams. The mass in grams is 6.02 with 10 being. We need to find the number of co2 molecule in 44 grams. Calculate the molar mass of co2: 4.4 g of co2 = x mole of co2. 8 people found it helpful. X = 0.1 mole of co2 gas. 44 g. Flask Contains 4.4 G Of.
From www.chegg.com
Solved CHEMWORK At a particular temperature a 2.00L flask Flask Contains 4.4 G Of 44 g ( molecular mass) of co2 = 1 mole of co2. (b)how many molecules of c o 2 gas are. Calculate the molar mass of co2: 4.4 g of co2 = x mole of co2. Let's take a look at this question. Calculate (b) how many moles of c o 2 gas does it contain? A flask contains 4.4g. Flask Contains 4.4 G Of.
From www.numerade.com
SOLVED A 100.0mL flask contains 0.250 g of a volatile oxide of Flask Contains 4.4 G Of Now, calculate the number of moles of co2 you have: We know, molar mass of carbon dioxide = 44 grams. Let's take a look at this question. That means 1 mole of carbon dioxide = 44. Calculate the molar mass of co2: A flask contains 4.4g of c o 2 gas. Calculate (b) how many moles of c o 2. Flask Contains 4.4 G Of.
From www.chegg.com
Solved (1) A 147−mL flask contains 0.500 g of a volatilized Flask Contains 4.4 G Of 4.4 g of co2 = x mole of co2. Let's take a look at this question. (b)how many molecules of c o 2 gas are. 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by avogadro's number to get. We know, molar mass of carbon dioxide = 44 grams. 44 g ( molecular mass) of co2 = 1 mole. Flask Contains 4.4 G Of.
From www.numerade.com
SOLVED Nitrogen and hydrogen gas react to form ammonia according to Flask Contains 4.4 G Of Calculate the molar mass of co2: 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by avogadro's number to get. We need to find the number of co2 molecule in 44 grams. Now, calculate the number of moles of co2 you have: A flask contains 4.4g of c o 2 gas. The mass in grams is 6.02 with 10. Flask Contains 4.4 G Of.
From www.numerade.com
A flask contains 0.25 mole of SO2(g), 0.50 mole of CH4(g), and 0.50 Flask Contains 4.4 G Of Let's take a look at this question. C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) = 44.01 g/mol. 4.4 g of co2 = x mole of co2. Now, calculate the number of moles of co2 you have: X = 0.1 mole of co2 gas. We know, molar mass of carbon. Flask Contains 4.4 G Of.
From www.numerade.com
SOLVED Three 7L flasks; fixed with pressure gauges and small valves Flask Contains 4.4 G Of Let's take a look at this question. Calculate the molar mass of co2: 4.4 g of co2 = x mole of co2. (b)how many molecules of c o 2 gas are. We know, molar mass of carbon dioxide = 44 grams. 44 g ( molecular mass) of co2 = 1 mole of co2. 4.4 g / 44.0087 g/mol = 0.099980231. Flask Contains 4.4 G Of.
From www.chegg.com
Solved A flask contains three gasses. Flask volume is 5.00 L Flask Contains 4.4 G Of 44 g ( molecular mass) of co2 = 1 mole of co2. We know, molar mass of carbon dioxide = 44 grams. A flask contains 4.4g of c o 2 gas. The mass in grams is 6.02 with 10 being. Now, calculate the number of moles of co2 you have: Calculate the molar mass of co2: 4.4 g / 44.0087. Flask Contains 4.4 G Of.
From www.vrogue.co
How To Use A Volumetric Flask vrogue.co Flask Contains 4.4 G Of Calculate the molar mass of co2: X = 0.1 mole of co2 gas. 4.4 g of co2 = x mole of co2. 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by avogadro's number to get. Calculate the molar mass of co2: Let's take a look at this question. Calculate (b) how many moles of c o 2 gas. Flask Contains 4.4 G Of.
From www.chegg.com
Solved The flask shown here contains 0.504 g of acid and a Flask Contains 4.4 G Of Calculate the molar mass of co2: Now, calculate the number of moles of co2 you have: 4.4 g of co2 = x mole of co2. X = 0.1 mole of co2 gas. 44 g ( molecular mass) of co2 = 1 mole of co2. A flask contains 4.4g of c o 2 gas. C = 12.01 g/mol o = 16.00. Flask Contains 4.4 G Of.
From www.alamy.com
Glass laboratory flask Stock Vector Images Alamy Flask Contains 4.4 G Of 44 g ( molecular mass) of co2 = 1 mole of co2. 8 people found it helpful. A flask contains 4.4g of c o 2 gas. (b)how many molecules of c o 2 gas are. Calculate the molar mass of co2: 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by avogadro's number to get. Calculate the molar mass. Flask Contains 4.4 G Of.
From www.chegg.com
Solved The flask shown here contains 10.0 mL of HCI and a Flask Contains 4.4 G Of Now, calculate the number of moles of co2 you have: 8 people found it helpful. C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) = 44.01 g/mol. Calculate the molar mass of co2: That means 1 mole of carbon dioxide = 44. Calculate the molar mass of co2: Let's take a. Flask Contains 4.4 G Of.
From www.numerade.com
SOLVEDA flask with a volume of 10.0 L contains 0.400 g of hydrogen gas Flask Contains 4.4 G Of Calculate the molar mass of co2: Let's take a look at this question. The mass in grams is 6.02 with 10 being. 8 people found it helpful. X = 0.1 mole of co2 gas. 44 g ( molecular mass) of co2 = 1 mole of co2. Now, calculate the number of moles of co2 you have: Calculate the molar mass. Flask Contains 4.4 G Of.
From www.chegg.com
Solved The flask shown here contains 10.0 mL of HCl and a Flask Contains 4.4 G Of We need to find the number of co2 molecule in 44 grams. 8 people found it helpful. 4.4 g of co2 = x mole of co2. Let's take a look at this question. C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) = 44.01 g/mol. A flask contains 4.4g of c. Flask Contains 4.4 G Of.
From www.bartleby.com
Answered If a flask contains a mixture of… bartleby Flask Contains 4.4 G Of 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by avogadro's number to get. We know, molar mass of carbon dioxide = 44 grams. C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) = 44.01 g/mol. 4.4 g of co2 = x mole of co2. We need to find the. Flask Contains 4.4 G Of.
From www.chegg.com
Solved 2) You have three identical flasks Flask A contains Flask Contains 4.4 G Of That means 1 mole of carbon dioxide = 44. A flask contains 4.4g of c o 2 gas. X = 0.1 mole of co2 gas. Calculate the molar mass of co2: 4.4 g of co2 = x mole of co2. (b)how many molecules of c o 2 gas are. 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by. Flask Contains 4.4 G Of.
From www.chegg.com
Solved The flask shown here contains 10.0 mL of HCI and a Flask Contains 4.4 G Of (b)how many molecules of c o 2 gas are. A flask contains 4.4g of c o 2 gas. Let's take a look at this question. Now, calculate the number of moles of co2 you have: 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by avogadro's number to get. 4.4 g of co2 = x mole of co2. That. Flask Contains 4.4 G Of.
From www.alamy.com
Rb flask hires stock photography and images Alamy Flask Contains 4.4 G Of Now, calculate the number of moles of co2 you have: Calculate the molar mass of co2: The mass in grams is 6.02 with 10 being. 44 g ( molecular mass) of co2 = 1 mole of co2. Let's take a look at this question. C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2. Flask Contains 4.4 G Of.
From www.numerade.com
SOLVEDTwo flasks of equal volume and at the same temperature contain Flask Contains 4.4 G Of X = 0.1 mole of co2 gas. Calculate the molar mass of co2: C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) = 44.01 g/mol. Now, calculate the number of moles of co2 you have: (b)how many molecules of c o 2 gas are. Let's take a look at this question.. Flask Contains 4.4 G Of.
From sse-kh.com
Conical Flask SSE Co.,LTD Flask Contains 4.4 G Of Calculate the molar mass of co2: Now, calculate the number of moles of co2 you have: The mass in grams is 6.02 with 10 being. 8 people found it helpful. A flask contains 4.4g of c o 2 gas. We know, molar mass of carbon dioxide = 44 grams. (b)how many molecules of c o 2 gas are. X =. Flask Contains 4.4 G Of.
From www.numerade.com
SOLVED A flask contains a mixture of two gases NO2 and Ne. The flask Flask Contains 4.4 G Of 8 people found it helpful. That means 1 mole of carbon dioxide = 44. Calculate the molar mass of co2: X = 0.1 mole of co2 gas. We need to find the number of co2 molecule in 44 grams. 44 g ( molecular mass) of co2 = 1 mole of co2. Calculate (b) how many moles of c o 2. Flask Contains 4.4 G Of.
From www.purplemoonpromo.co.uk
1litrevacuumflaskdemo Purple Moon Flask Contains 4.4 G Of (b)how many molecules of c o 2 gas are. 44 g ( molecular mass) of co2 = 1 mole of co2. Calculate the molar mass of co2: 8 people found it helpful. X = 0.1 mole of co2 gas. Now, calculate the number of moles of co2 you have: 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by. Flask Contains 4.4 G Of.
From www.chegg.com
Solved The flask shown here contains 0.238 g of acid and a Flask Contains 4.4 G Of We know, molar mass of carbon dioxide = 44 grams. A flask contains 4.4g of c o 2 gas. Let's take a look at this question. (b)how many molecules of c o 2 gas are. Now, calculate the number of moles of co2 you have: That means 1 mole of carbon dioxide = 44. Calculate the molar mass of co2:. Flask Contains 4.4 G Of.
From byjus.com
11. Two flasks X and Y of volumes 250 ml and 300 ml respectively at the Flask Contains 4.4 G Of 44 g ( molecular mass) of co2 = 1 mole of co2. (b)how many molecules of c o 2 gas are. Calculate (b) how many moles of c o 2 gas does it contain? That means 1 mole of carbon dioxide = 44. C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x. Flask Contains 4.4 G Of.
From www.indiamart.com
Dolphin Labware Spherical Round Bottom Glass Flask, For Chemical Flask Contains 4.4 G Of We know, molar mass of carbon dioxide = 44 grams. (b)how many molecules of c o 2 gas are. 4.4 g of co2 = x mole of co2. X = 0.1 mole of co2 gas. Calculate the molar mass of co2: C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) =. Flask Contains 4.4 G Of.
From www.chegg.com
Solved The flask shown here contains 0.231 g of acid and a Flask Contains 4.4 G Of C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) = 44.01 g/mol. A flask contains 4.4g of c o 2 gas. The mass in grams is 6.02 with 10 being. X = 0.1 mole of co2 gas. Calculate the molar mass of co2: Now, calculate the number of moles of co2. Flask Contains 4.4 G Of.
From www.craiyon.com
Blue gradient liquid in a flask on Craiyon Flask Contains 4.4 G Of (b)how many molecules of c o 2 gas are. We need to find the number of co2 molecule in 44 grams. Calculate the molar mass of co2: C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) = 44.01 g/mol. 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by avogadro's. Flask Contains 4.4 G Of.
From www.pinterest.com
A Visual Guide to Chemistry Glassware Flask design, Chemistry Flask Contains 4.4 G Of 4.4 g / 44.0087 g/mol = 0.099980231 mol finally, multiply by avogadro's number to get. 8 people found it helpful. Calculate (b) how many moles of c o 2 gas does it contain? (b)how many molecules of c o 2 gas are. We need to find the number of co2 molecule in 44 grams. Calculate the molar mass of co2:. Flask Contains 4.4 G Of.
From www.numerade.com
SOLVED A 250.0mL flask contains 0.2500 g of a volatile oxide of Flask Contains 4.4 G Of Let's take a look at this question. Calculate the molar mass of co2: C = 12.01 g/mol o = 16.00 g/mol molar mass of co2 = 12.01 + (2 x 16.00) = 44.01 g/mol. 8 people found it helpful. Now, calculate the number of moles of co2 you have: 4.4 g of co2 = x mole of co2. 4.4 g. Flask Contains 4.4 G Of.