How To Get Overall Rate Law . At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. Rate net = rate forward + rate reverse. Use rate laws to calculate reaction rates. In order to experimentally determine a rate law, a series of experiments must be performed with various starting concentrations. The rate law will be \[rate = [a]^a[b]^b. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it. Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. Use rate and concentration data to identify. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate. Explain the form and function of a rate law.
from www.chegg.com
The rate law will be \[rate = [a]^a[b]^b. Rate net = rate forward + rate reverse. Explain the form and function of a rate law. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate. Use rate laws to calculate reaction rates. Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. Use rate and concentration data to identify. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: In order to experimentally determine a rate law, a series of experiments must be performed with various starting concentrations.
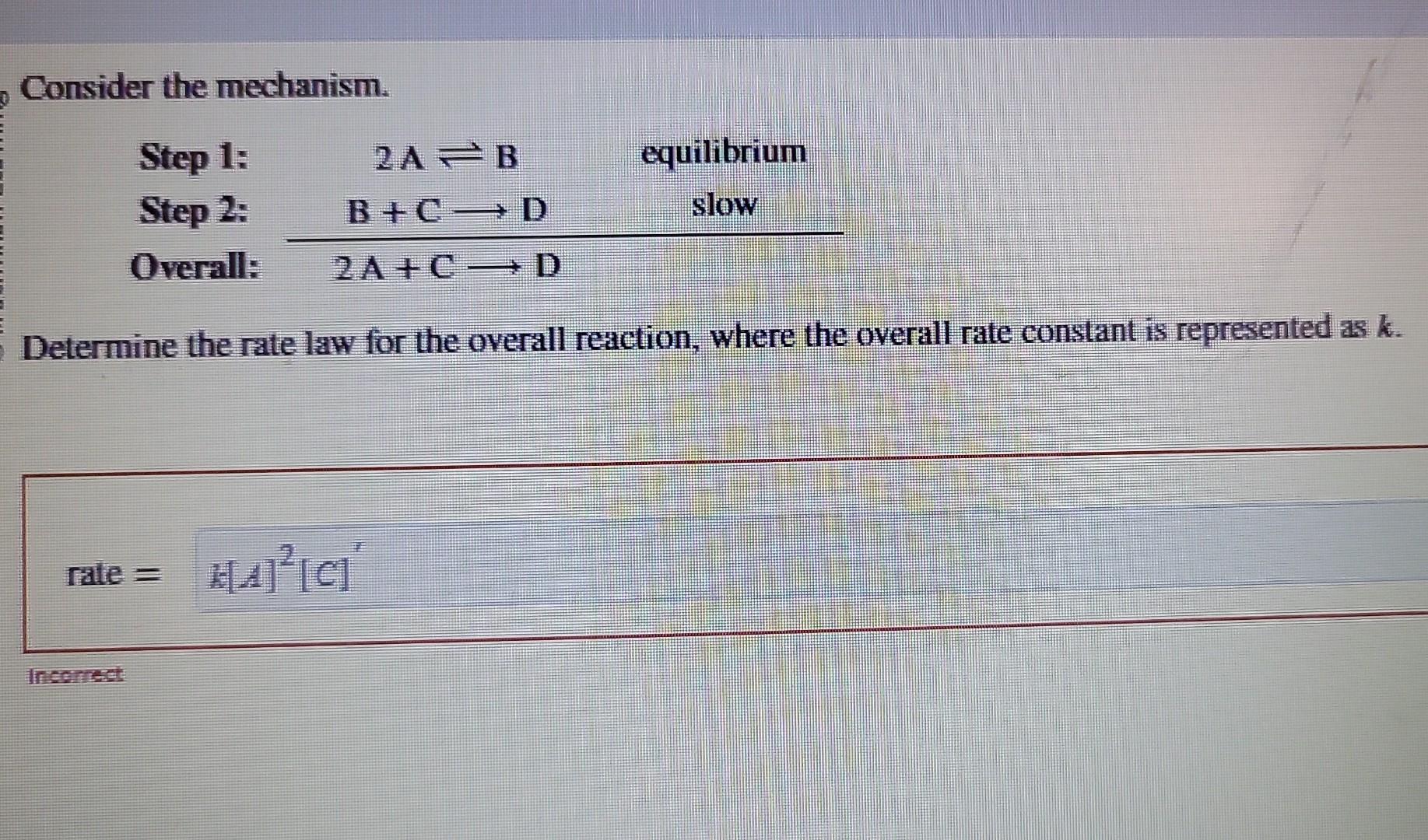
Solved Consider the mechanism. Determine the rate law for
How To Get Overall Rate Law The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it. Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: Rate net = rate forward + rate reverse. Explain the form and function of a rate law. In order to experimentally determine a rate law, a series of experiments must be performed with various starting concentrations. Use rate laws to calculate reaction rates. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate. The rate law will be \[rate = [a]^a[b]^b. Use rate and concentration data to identify. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it.
From warreninstitute.org
MASTER Reaction Rates Integrated Rate Laws UNVEILED How To Get Overall Rate Law At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it. 2n 2 o. How To Get Overall Rate Law.
From mavink.com
Rate Constant Calculation How To Get Overall Rate Law At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it. Rate net =. How To Get Overall Rate Law.
From www.chegg.com
Rate law and rate constant, k Write the rate law for How To Get Overall Rate Law The rate law will be \[rate = [a]^a[b]^b. Use rate and concentration data to identify. In order to experimentally determine a rate law, a series of experiments must be performed with various starting concentrations. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: Rate = k [n 2 o. How To Get Overall Rate Law.
From www.youtube.com
Determining Rate Laws from Experimental Data YouTube How To Get Overall Rate Law In order to experimentally determine a rate law, a series of experiments must be performed with various starting concentrations. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: Explain the form and function of a rate law. Rate net = rate forward + rate reverse. Use rate and concentration. How To Get Overall Rate Law.
From www.chegg.com
Solved Part A What is the rate law for the following How To Get Overall Rate Law Use rate laws to calculate reaction rates. At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. The rate law will be \[rate = [a]^a[b]^b. Use rate and concentration data to identify. Explain the form and function of a rate law. In order to experimentally determine a rate. How To Get Overall Rate Law.
From brainly.com
Determine the overall order of reaction to which the following rate law How To Get Overall Rate Law At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. The rate law will be \[rate = [a]^a[b]^b. In order to experimentally determine a rate law, a series of experiments must be. How To Get Overall Rate Law.
From study.com
How to Determine the Order of Reaction by Comparing Initial Rates of How To Get Overall Rate Law Use rate laws to calculate reaction rates. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate. Rate net = rate forward + rate reverse. Use rate and concentration data to identify. Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. Explain the form and function of. How To Get Overall Rate Law.
From www.chegg.com
Solved Determine the rate law for the overall reaction. How To Get Overall Rate Law At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. Rate net = rate forward + rate reverse. In order to experimentally determine a rate law, a series of experiments must be performed with various starting concentrations. Explain the form and function of a rate law. 2n 2. How To Get Overall Rate Law.
From www.sliderbase.com
Rate Laws Presentation Chemistry How To Get Overall Rate Law The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: Explain the form and function. How To Get Overall Rate Law.
From www.slideserve.com
PPT PowerPoint Presentation ID3920002 How To Get Overall Rate Law The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it. In order to experimentally determine a rate law, a series of experiments must be performed with various starting concentrations. Rate = k [n 2 o. How To Get Overall Rate Law.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical How To Get Overall Rate Law Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. Use rate and concentration data to identify. Use rate laws to calculate reaction rates. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: Since the rate of a reaction has the dimensions of. How To Get Overall Rate Law.
From www.slideserve.com
PPT Chemical Rate Laws ORDER OF REACTION PowerPoint How To Get Overall Rate Law Use rate and concentration data to identify. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate. Use rate laws to calculate reaction rates. Rate net = rate forward + rate reverse. At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant.. How To Get Overall Rate Law.
From oneclass.com
OneClass Rate law and overall reaction order. I cannt figure out how How To Get Overall Rate Law 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: Rate net = rate forward + rate reverse. Use rate and concentration data to identify. Use rate laws to calculate reaction rates. Explain the form and function of a rate law. Rate = k [n 2 o 5 (g)] state. How To Get Overall Rate Law.
From www.numerade.com
SOLVED Use the experimental data given in the table and determine the How To Get Overall Rate Law Rate net = rate forward + rate reverse. At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. Use rate and concentration data to identify. The rate law will be \[rate =. How To Get Overall Rate Law.
From www.youtube.com
16.1/R2.2.6 Rate constant, overall order of reaction, order of reaction How To Get Overall Rate Law The rate law will be \[rate = [a]^a[b]^b. Use rate laws to calculate reaction rates. At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. Rate net = rate forward + rate reverse. Use rate and concentration data to identify. 2n 2 o 5 (g) → 4no 2. How To Get Overall Rate Law.
From www.chegg.com
Solved Consider the following mechanism. Determine the rate How To Get Overall Rate Law Rate net = rate forward + rate reverse. In order to experimentally determine a rate law, a series of experiments must be performed with various starting concentrations. Use rate and concentration data to identify. The rate law will be \[rate = [a]^a[b]^b. At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent. How To Get Overall Rate Law.
From www.slideserve.com
PPT Rate Laws PowerPoint Presentation, free download ID4905523 How To Get Overall Rate Law Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it. Since the rate of a reaction has the dimensions. How To Get Overall Rate Law.
From www.slideserve.com
PPT Rate Laws PowerPoint Presentation, free download ID4206487 How To Get Overall Rate Law The rate law will be \[rate = [a]^a[b]^b. Use rate and concentration data to identify. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate. Explain the form and function of a rate law. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship. How To Get Overall Rate Law.
From shilohgrohuerta.blogspot.com
Rate of Reaction Calculation How To Get Overall Rate Law Use rate and concentration data to identify. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. Rate net = rate forward + rate reverse. In order to experimentally determine a rate law, a. How To Get Overall Rate Law.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial How To Get Overall Rate Law The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it. At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. Use rate laws. How To Get Overall Rate Law.
From general.chemistrysteps.com
Rate Law and Reaction Order Chemistry Steps How To Get Overall Rate Law 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. Explain the form and function of a rate law. Rate net = rate forward + rate reverse. At equilibrium, rate net 0 and the. How To Get Overall Rate Law.
From www.numerade.com
SOLVEDSTEP 1 A +B > C slow STEP 2 A + C > D fast overall 2A How To Get Overall Rate Law The rate law will be \[rate = [a]^a[b]^b. Rate net = rate forward + rate reverse. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate. Explain the form and function of a rate law. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a. How To Get Overall Rate Law.
From www.youtube.com
Reaction Mechanisms and rate laws elementary steps and overall How To Get Overall Rate Law Use rate and concentration data to identify. Use rate laws to calculate reaction rates. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. The rate law will be \[rate = [a]^a[b]^b. In order. How To Get Overall Rate Law.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical How To Get Overall Rate Law Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. Use rate laws to calculate reaction rates. In order to experimentally determine a rate law, a series of experiments must be performed with various starting concentrations. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this. How To Get Overall Rate Law.
From www.youtube.com
DAT Rate Law and Rate Determining Step (+ Examples) YouTube How To Get Overall Rate Law At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: The rate law will be \[rate = [a]^a[b]^b. The rate law (also known as the rate equation) for. How To Get Overall Rate Law.
From irenenewssalinas.blogspot.com
How to Calculate Overall Yield of a Multistep Reaction How To Get Overall Rate Law At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. Use rate laws to calculate reaction rates. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: The rate law will be \[rate = [a]^a[b]^b. Rate = k. How To Get Overall Rate Law.
From www.youtube.com
Determining the Rate Law for a Mechanism with a Fast Equilibrium Step How To Get Overall Rate Law The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it. At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. Rate = k. How To Get Overall Rate Law.
From www.slideserve.com
PPT The Rate Law PowerPoint Presentation, free download ID6555772 How To Get Overall Rate Law Rate net = rate forward + rate reverse. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: Explain the form and function of a rate law. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate. Use rate laws to calculate reaction rates.. How To Get Overall Rate Law.
From www.vrogue.co
Solved Part B What Is The Rate Constant Of A First Or vrogue.co How To Get Overall Rate Law Explain the form and function of a rate law. At equilibrium, rate net 0 and the rate law must reduce to an equation that is thermodynamically consistent with the equilibrium constant. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate. Rate = k [n 2 o 5 (g)] state the order of reaction. How To Get Overall Rate Law.
From www.chegg.com
Solved Consider the mechanism. Determine the rate law for How To Get Overall Rate Law The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it. In order to experimentally determine a rate law, a series of experiments must be performed with various starting concentrations. Explain the form and function of. How To Get Overall Rate Law.
From www.slideshare.net
8.1 rate law How To Get Overall Rate Law Explain the form and function of a rate law. Rate = k [n 2 o 5 (g)] state the order of reaction with respect to. Use rate and concentration data to identify. The rate law will be \[rate = [a]^a[b]^b. Use rate laws to calculate reaction rates. At equilibrium, rate net 0 and the rate law must reduce to an. How To Get Overall Rate Law.
From www.slideserve.com
PPT Integrated Rate Law PowerPoint Presentation, free download ID How To Get Overall Rate Law In order to experimentally determine a rate law, a series of experiments must be performed with various starting concentrations. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate. 2n 2 o 5 (g) → 4no 2 (g) + o 2 (g) the rate equation for this reaction is: Rate net = rate forward. How To Get Overall Rate Law.
From www.youtube.com
Rate Law for a Mechanism with a Fast Initial Step YouTube How To Get Overall Rate Law The rate law will be \[rate = [a]^a[b]^b. Use rate laws to calculate reaction rates. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it. Since the rate of a reaction has the dimensions of. How To Get Overall Rate Law.
From www.slideserve.com
PPT Chemical Rate Laws ORDER OF REACTION PowerPoint How To Get Overall Rate Law Rate net = rate forward + rate reverse. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it. 2n. How To Get Overall Rate Law.
From www.wizeprep.com
The Rate Law Wize University Chemistry Textbook Wizeprep How To Get Overall Rate Law Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate. Rate net = rate forward + rate reverse. In order to experimentally determine a rate law, a series of experiments must be performed with various starting concentrations. The rate law will be \[rate = [a]^a[b]^b. The rate law (also known as the rate equation). How To Get Overall Rate Law.