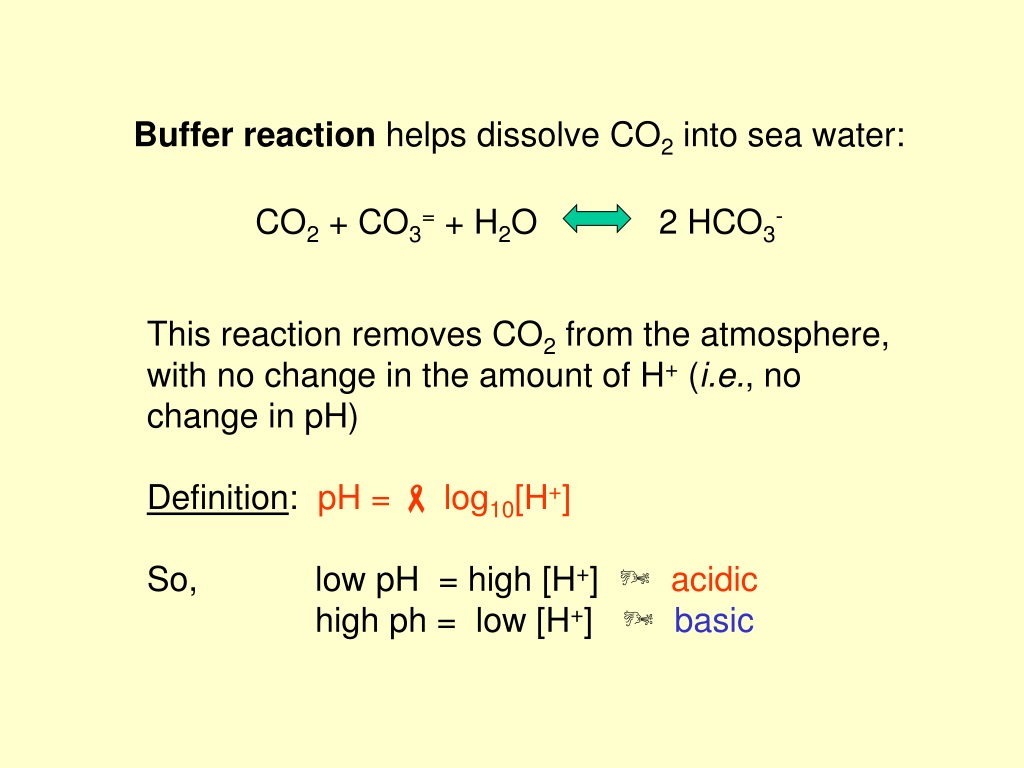
Buffer Reaction Example . Similarly, adding a base like sodium hydroxide (naoh) results in the acetic. Hf buffer in this example we will continue to use the hydrofluoric acid buffer. In this case, if the solution contained equal molar concentrations of. a common example would be a mixture of ethanoic acid and sodium ethanoate in solution. When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. many chemical reactions are carried out at a constant ph. for example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). In nature, there are many systems that use buffering for ph. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14). another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). We will discuss the process for preparing a buffer of hf at a ph of 3.0. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl).
from www.slideserve.com
buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14). Similarly, adding a base like sodium hydroxide (naoh) results in the acetic. Hf buffer in this example we will continue to use the hydrofluoric acid buffer. In this case, if the solution contained equal molar concentrations of. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). In nature, there are many systems that use buffering for ph. We will discuss the process for preparing a buffer of hf at a ph of 3.0. many chemical reactions are carried out at a constant ph. for example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona).
PPT Chapter 8—Part 2 The Carbon Cycle/ Marine Organic
Buffer Reaction Example buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14). Similarly, adding a base like sodium hydroxide (naoh) results in the acetic. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14). a common example would be a mixture of ethanoic acid and sodium ethanoate in solution. When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). In nature, there are many systems that use buffering for ph. Hf buffer in this example we will continue to use the hydrofluoric acid buffer. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). We will discuss the process for preparing a buffer of hf at a ph of 3.0. In this case, if the solution contained equal molar concentrations of. for example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). many chemical reactions are carried out at a constant ph.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Buffer Reaction Example When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. Hf buffer in this example we will continue to use the hydrofluoric acid buffer. many chemical reactions are carried out at a constant ph. a common example would be a mixture of. Buffer Reaction Example.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Buffer Reaction Example buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14). We will discuss the process for preparing a buffer of hf at a ph of 3.0. Hf buffer in this example we will continue to use the hydrofluoric acid buffer. for example, consider a buffer made. Buffer Reaction Example.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy Buffer Reaction Example In nature, there are many systems that use buffering for ph. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). for example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with. Buffer Reaction Example.
From www.slideshare.net
Lect w9 152 buffers and ksp_alg Buffer Reaction Example When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. In this case, if the solution contained equal molar concentrations of. In nature, there are many systems that use buffering for ph. buffer solutions resist a change in ph when small amounts of. Buffer Reaction Example.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Buffer Reaction Example Hf buffer in this example we will continue to use the hydrofluoric acid buffer. In this case, if the solution contained equal molar concentrations of. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). In nature, there are many systems that use buffering for ph. Similarly, adding. Buffer Reaction Example.
From study.com
Buffer System in Chemistry Definition, Function & Examples Lesson Buffer Reaction Example for example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). In this case, if the solution contained equal molar concentrations of. Hf buffer in this example we will continue to use the hydrofluoric acid buffer. a common example would be a mixture of ethanoic acid and sodium ethanoate in solution. In nature, there are. Buffer Reaction Example.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Buffer Reaction Example Similarly, adding a base like sodium hydroxide (naoh) results in the acetic. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14). another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). We will discuss. Buffer Reaction Example.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Reaction Example a common example would be a mixture of ethanoic acid and sodium ethanoate in solution. When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. In nature, there are many systems that use buffering for ph. In this case, if the solution contained. Buffer Reaction Example.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Buffer Reaction Example In this case, if the solution contained equal molar concentrations of. When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. We will discuss the process for preparing a buffer of hf at a ph of 3.0. for example, consider a buffer made. Buffer Reaction Example.
From giorjqagn.blob.core.windows.net
Buffer With Example at Javier Rodriquez blog Buffer Reaction Example another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. In nature, there are many systems that use buffering for ph. Hf. Buffer Reaction Example.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Buffer Reaction Example a common example would be a mixture of ethanoic acid and sodium ethanoate in solution. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). In this case, if the solution contained equal molar concentrations of. When hydrochloric acid (hcl) is. Buffer Reaction Example.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co Buffer Reaction Example Similarly, adding a base like sodium hydroxide (naoh) results in the acetic. Hf buffer in this example we will continue to use the hydrofluoric acid buffer. In this case, if the solution contained equal molar concentrations of. In nature, there are many systems that use buffering for ph. We will discuss the process for preparing a buffer of hf at. Buffer Reaction Example.
From giobxirow.blob.core.windows.net
Organic Buffer Examples at Steven Russell blog Buffer Reaction Example When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. In nature, there are many systems that use buffering for ph. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14).. Buffer Reaction Example.
From www.slideshare.net
2012 topic 18 2 buffer solutions Buffer Reaction Example We will discuss the process for preparing a buffer of hf at a ph of 3.0. When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium. Buffer Reaction Example.
From www.youtube.com
buffer actions electrochemistry class 12 chemistry subject notes Buffer Reaction Example another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. We will discuss the process for. Buffer Reaction Example.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Reaction Example for example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). In nature, there are many systems that use buffering for ph. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14). In this case, if the solution contained equal molar concentrations of.. Buffer Reaction Example.
From giokrstgg.blob.core.windows.net
Buffer Solution Equilibrium at Jacqueline Scott blog Buffer Reaction Example another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). In nature, there are many systems that use buffering for ph. When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid. Buffer Reaction Example.
From www.peoi.org
Chapter 12 Section G Buffers Buffer Reaction Example Similarly, adding a base like sodium hydroxide (naoh) results in the acetic. We will discuss the process for preparing a buffer of hf at a ph of 3.0. In this case, if the solution contained equal molar concentrations of. for example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). In nature, there are many systems. Buffer Reaction Example.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation Buffer Reaction Example We will discuss the process for preparing a buffer of hf at a ph of 3.0. many chemical reactions are carried out at a constant ph. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14). In nature, there are many systems that use buffering for. Buffer Reaction Example.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Buffer Reaction Example We will discuss the process for preparing a buffer of hf at a ph of 3.0. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14). another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4. Buffer Reaction Example.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Buffer Reaction Example buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14). another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). Hf buffer in this example we will continue to. Buffer Reaction Example.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Reaction Example buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14). In this case, if the solution contained equal molar concentrations of. a common example would be a mixture of ethanoic acid and sodium ethanoate in solution. another example of a buffer is a solution containing. Buffer Reaction Example.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation Buffer Reaction Example Hf buffer in this example we will continue to use the hydrofluoric acid buffer. Similarly, adding a base like sodium hydroxide (naoh) results in the acetic. a common example would be a mixture of ethanoic acid and sodium ethanoate in solution. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium. Buffer Reaction Example.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Reaction Example In nature, there are many systems that use buffering for ph. In this case, if the solution contained equal molar concentrations of. Hf buffer in this example we will continue to use the hydrofluoric acid buffer. a common example would be a mixture of ethanoic acid and sodium ethanoate in solution. another example of a buffer is a. Buffer Reaction Example.
From mungfali.com
NH3 And NH4Cl Buffer Equation Buffer Reaction Example Similarly, adding a base like sodium hydroxide (naoh) results in the acetic. In nature, there are many systems that use buffering for ph. many chemical reactions are carried out at a constant ph. a common example would be a mixture of ethanoic acid and sodium ethanoate in solution. Hf buffer in this example we will continue to use. Buffer Reaction Example.
From www.youtube.com
Intro to Buffers and the HendersonHasselbalch Equation YouTube Buffer Reaction Example buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 14.14). In this case, if the solution contained equal molar concentrations of. We will discuss the process for preparing a buffer of hf at a ph of 3.0. Hf buffer in this example we will continue to use. Buffer Reaction Example.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination for Biologists Buffer Reaction Example We will discuss the process for preparing a buffer of hf at a ph of 3.0. In this case, if the solution contained equal molar concentrations of. When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. buffer solutions resist a change in. Buffer Reaction Example.
From www.slideserve.com
PPT Chapter 8—Part 2 The Carbon Cycle/ Marine Organic Buffer Reaction Example another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). We will discuss the process for preparing a buffer of. Buffer Reaction Example.
From www.ck12.org
Buffer Solutions Example 1 ( Video ) Chemistry CK12 Foundation Buffer Reaction Example When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. a common example would be a mixture of ethanoic acid and sodium ethanoate in solution. for example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). another example. Buffer Reaction Example.
From www.slideshare.net
02 hydrolysis. buffers__colloids Buffer Reaction Example In this case, if the solution contained equal molar concentrations of. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). Hf buffer in this example we will continue to use the hydrofluoric acid buffer. When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the. Buffer Reaction Example.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Buffer Reaction Example Similarly, adding a base like sodium hydroxide (naoh) results in the acetic. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl). In this case, if the solution contained equal molar concentrations of. many chemical reactions are carried out at a constant ph. another example of. Buffer Reaction Example.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co Buffer Reaction Example When hydrochloric acid (hcl) is added, the acetate ion (ch₃coo⁻) reacts with the h⁺ ions from hcl, forming more acetic acid and mitigating the ph change. We will discuss the process for preparing a buffer of hf at a ph of 3.0. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium. Buffer Reaction Example.
From acsessible.github.io
Acid Buffer ACcessible School Buffer Reaction Example Similarly, adding a base like sodium hydroxide (naoh) results in the acetic. In nature, there are many systems that use buffering for ph. We will discuss the process for preparing a buffer of hf at a ph of 3.0. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4. Buffer Reaction Example.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Buffer Reaction Example Hf buffer in this example we will continue to use the hydrofluoric acid buffer. another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). In nature, there are many systems that use buffering for ph. many chemical reactions are carried out. Buffer Reaction Example.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Reaction Example another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). In nature, there are many systems that use buffering for ph. many chemical reactions are carried out at a constant ph. another example of a buffer is a solution containing. Buffer Reaction Example.