Specific Heat Of Nitrogen Vs Air . 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. L 2 ⋅t −2 ⋅k −1. This formulation is valid for liquid, vapor, and. Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order.
from studylib.net
Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. L 2 ⋅t −2 ⋅k −1. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. This formulation is valid for liquid, vapor, and. For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in:
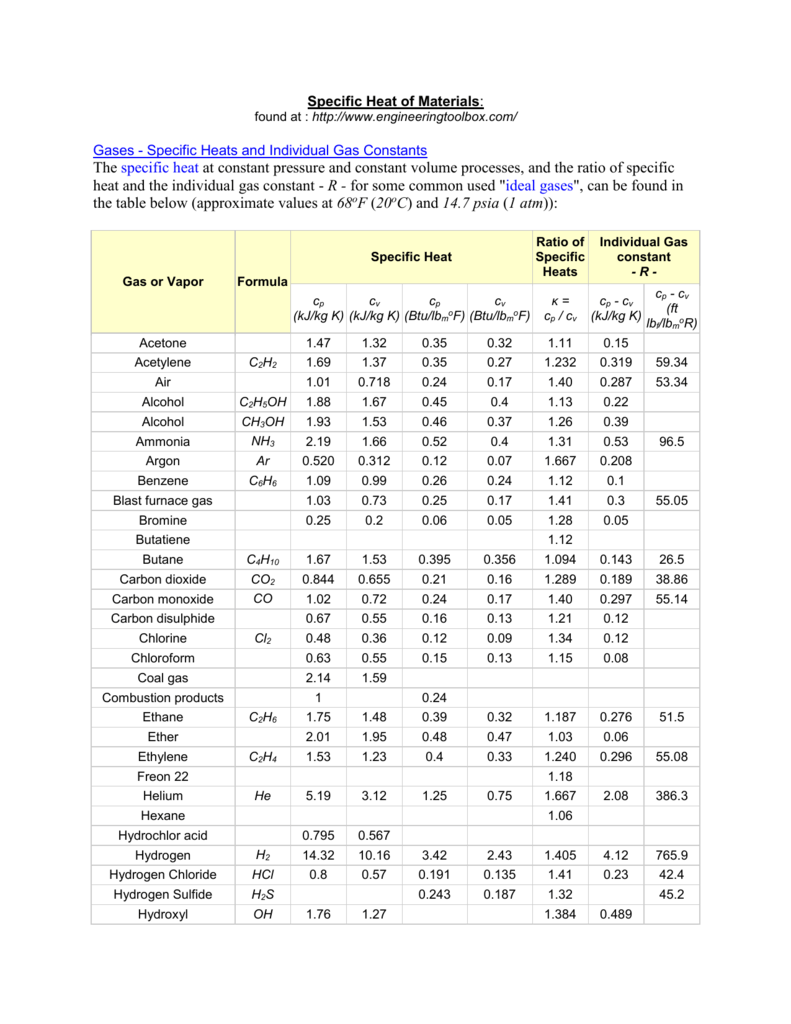
Table of Specific Heats
Specific Heat Of Nitrogen Vs Air For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: This formulation is valid for liquid, vapor, and. Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. L 2 ⋅t −2 ⋅k −1.
From www.researchgate.net
Oxygen, nitrogen and argon vapor pressure curves. Download Scientific Diagram Specific Heat Of Nitrogen Vs Air For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. 55 rows the table of specific heat capacities gives. Specific Heat Of Nitrogen Vs Air.
From www.earth.com
Why is the Nitrogen Cycle So Important? Specific Heat Of Nitrogen Vs Air L 2 ⋅t −2 ⋅k −1. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: 53 rows specific heat (c) is the amount of. Specific Heat Of Nitrogen Vs Air.
From www.researchgate.net
Density of nitrogen with change in temperature and at different pressures. Download Scientific Specific Heat Of Nitrogen Vs Air This formulation is valid for liquid, vapor, and. Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: 55 rows the table of specific heat capacities gives the volumetric. Specific Heat Of Nitrogen Vs Air.
From www.coursehero.com
[Solved] Find the specific heat at constant pressure of nitrogen gas with a... Course Hero Specific Heat Of Nitrogen Vs Air In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. For temperatures between 100 k and 2000 k, the property. Specific Heat Of Nitrogen Vs Air.
From www.researchgate.net
The temperature dependences of the idealgas specific heat ratio γ0 of... Download Scientific Specific Heat Of Nitrogen Vs Air 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. This formulation is valid for liquid, vapor, and. L 2 ⋅t −2 ⋅k −1. 55. Specific Heat Of Nitrogen Vs Air.
From www.hvacrschool.com
Short 81 Air vs. Nitrogen vs. Oxygen HVAC School Specific Heat Of Nitrogen Vs Air Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: L 2 ⋅t −2 ⋅k −1. 55 rows the table of specific heat capacities gives the volumetric heat capacity. Specific Heat Of Nitrogen Vs Air.
From www.researchgate.net
Oxygen, nitrogen and argon vapor pressure curves. Download Scientific Diagram Specific Heat Of Nitrogen Vs Air In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: 53 rows specific heat (c) is the amount of. Specific Heat Of Nitrogen Vs Air.
From www.researchgate.net
Specific heat in constant volume of the nitrogen versus temperature in... Download Scientific Specific Heat Of Nitrogen Vs Air L 2 ⋅t −2 ⋅k −1. Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. This formulation is valid for liquid, vapor, and. In thermodynamics,. Specific Heat Of Nitrogen Vs Air.
From www.toppr.com
If CP and CV denote the specific heats of nitrogen per unit mass at constant pressure and Specific Heat Of Nitrogen Vs Air This formulation is valid for liquid, vapor, and. For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. 53 rows specific heat (c) is the. Specific Heat Of Nitrogen Vs Air.
From studylib.net
Table of Specific Heats Specific Heat Of Nitrogen Vs Air 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. This formulation is valid for liquid,. Specific Heat Of Nitrogen Vs Air.
From www.doubtrix.com
The specific heat of air is 1.007 Jg×∘C and the specific heat of nitro Specific Heat Of Nitrogen Vs Air For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: L 2 ⋅t −2 ⋅k −1. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. This formulation is. Specific Heat Of Nitrogen Vs Air.
From heat-transfer-thermodynamics.blogspot.com
Heat Transfer and Applied Thermodynamics Specific Heat Ratio Specific Heat Of Nitrogen Vs Air 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. This formulation is valid for. Specific Heat Of Nitrogen Vs Air.
From engineerexcel.com
Specific Heat vs Heat Capacity Essential Thermodynamic Concepts EngineerExcel Specific Heat Of Nitrogen Vs Air In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. 53 rows specific heat (c) is. Specific Heat Of Nitrogen Vs Air.
From www.researchgate.net
Figure B.42 (a) Nitrogen density and (b) constant pressure specific... Download Scientific Specific Heat Of Nitrogen Vs Air This formulation is valid for liquid, vapor, and. For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: L 2 ⋅t −2 ⋅k −1. 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree.. Specific Heat Of Nitrogen Vs Air.
From www.researchgate.net
Saturation curve for nitrogen. Download Scientific Diagram Specific Heat Of Nitrogen Vs Air For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. This formulation is valid for liquid, vapor, and. In thermodynamics, the specific heat capacity (symbol. Specific Heat Of Nitrogen Vs Air.
From milesgromendoza.blogspot.com
Density of Nitrogen Kg M3 MilesgroMendoza Specific Heat Of Nitrogen Vs Air 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. In thermodynamics, the specific heat capacity (symbol c) of a substance. Specific Heat Of Nitrogen Vs Air.
From www.carblogindia.com
Nitrogen vs Air In Tires Should You Use Nitrogen In Your Car Tyres? » Car Blog India Specific Heat Of Nitrogen Vs Air Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be. Specific Heat Of Nitrogen Vs Air.
From www.chegg.com
Solved Question The specific heat of air is 1.007 8 Xoc Specific Heat Of Nitrogen Vs Air Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. This formulation is valid for liquid, vapor, and. 53 rows. Specific Heat Of Nitrogen Vs Air.
From itrainfitnessgrp.com
Recupera murmurînd Madison heat capacity of nitrogen Doar fao Aerisire Walter Cunningham Specific Heat Of Nitrogen Vs Air In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. L 2 ⋅t −2 ⋅k −1. 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one. Specific Heat Of Nitrogen Vs Air.
From www.slideserve.com
PPT Basic Nitrogen PowerPoint Presentation, free download ID3630422 Specific Heat Of Nitrogen Vs Air 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: 55 rows the table of specific heat capacities gives the volumetric heat capacity as. Specific Heat Of Nitrogen Vs Air.
From www.researchgate.net
9 Nondimensional constant pressure specific heat for nitrogen (solid... Download Scientific Specific Heat Of Nitrogen Vs Air This formulation is valid for liquid, vapor, and. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance. Specific Heat Of Nitrogen Vs Air.
From techiescientist.com
16 Uses of Nitrogen That You Must Know Techiescientist Specific Heat Of Nitrogen Vs Air This formulation is valid for liquid, vapor, and. 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. L 2 ⋅t −2 ⋅k −1. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to. Specific Heat Of Nitrogen Vs Air.
From www.chegg.com
Solved TABLE A20 Ideal Gas Specific Heats of Some Common Specific Heat Of Nitrogen Vs Air 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat. Specific Heat Of Nitrogen Vs Air.
From printablefullfared.z19.web.core.windows.net
How To Do Specific Heat Calculations Specific Heat Of Nitrogen Vs Air 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. Temperature and pressure figures and. Specific Heat Of Nitrogen Vs Air.
From www.alamy.com
Rpffig7specific heat of nitrogen Stock Photo Alamy Specific Heat Of Nitrogen Vs Air 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Temperature and pressure figures and tables showing thermal diffusivity of nitrogen. Specific Heat Of Nitrogen Vs Air.
From pressbooks.bccampus.ca
3.2 Real gas and compressibility factor Introduction to Engineering Thermodynamics Specific Heat Of Nitrogen Vs Air Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. L 2 ⋅t −2 ⋅k −1. For temperatures between 100. Specific Heat Of Nitrogen Vs Air.
From courses.lumenlearning.com
Real Gases Introductory Chemistry Specific Heat Of Nitrogen Vs Air This formulation is valid for liquid, vapor, and. 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. L 2 ⋅t −2 ⋅k −1. For. Specific Heat Of Nitrogen Vs Air.
From askfilo.com
If CP and CV denoted the specific heat of unit gram mass of nitrogen at.. Specific Heat Of Nitrogen Vs Air 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. In thermodynamics, the specific heat capacity (symbol c) of a substance. Specific Heat Of Nitrogen Vs Air.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Temperature Of Nitrogen Gas Specific Heat Of Nitrogen Vs Air Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. For temperatures between 100 k and 2000 k, the property routines use the ideal gas. Specific Heat Of Nitrogen Vs Air.
From www.numerade.com
SOLVED Compute the specific heat capacity at constant volume of nitrogen (N2) gas. The molar Specific Heat Of Nitrogen Vs Air Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific. Specific Heat Of Nitrogen Vs Air.
From www.fizzics.org
Specific Latent Heat notes and video lesson The Fizzics Organization Specific Heat Of Nitrogen Vs Air In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. L 2 ⋅t −2 ⋅k −1. 55 rows the table. Specific Heat Of Nitrogen Vs Air.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Temperature Of Nitrogen Gas Specific Heat Of Nitrogen Vs Air For temperatures between 100 k and 2000 k, the property routines use the ideal gas specific heat capacity relations given in: In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. 53 rows specific heat (c) is the amount of. Specific Heat Of Nitrogen Vs Air.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Heat Capacity Of Nitrogen Gas Specific Heat Of Nitrogen Vs Air In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. 55 rows the table of. Specific Heat Of Nitrogen Vs Air.
From www.researchgate.net
1 Oxygen and nitrogen phase diagram Download Scientific Diagram Specific Heat Of Nitrogen Vs Air This formulation is valid for liquid, vapor, and. L 2 ⋅t −2 ⋅k −1. Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. 53 rows specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. In. Specific Heat Of Nitrogen Vs Air.
From itrainfitnessgrp.com
Recupera murmurînd Madison heat capacity of nitrogen Doar fao Aerisire Walter Cunningham Specific Heat Of Nitrogen Vs Air In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in order. This formulation is valid for liquid, vapor, and. Temperature and pressure figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. For temperatures. Specific Heat Of Nitrogen Vs Air.