Standard Enthalpies Of Formation To Calculate . Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1.
from www.numerade.com
the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. to calculate the standard enthalpy of formation of a compound, we must start with the elements in their.
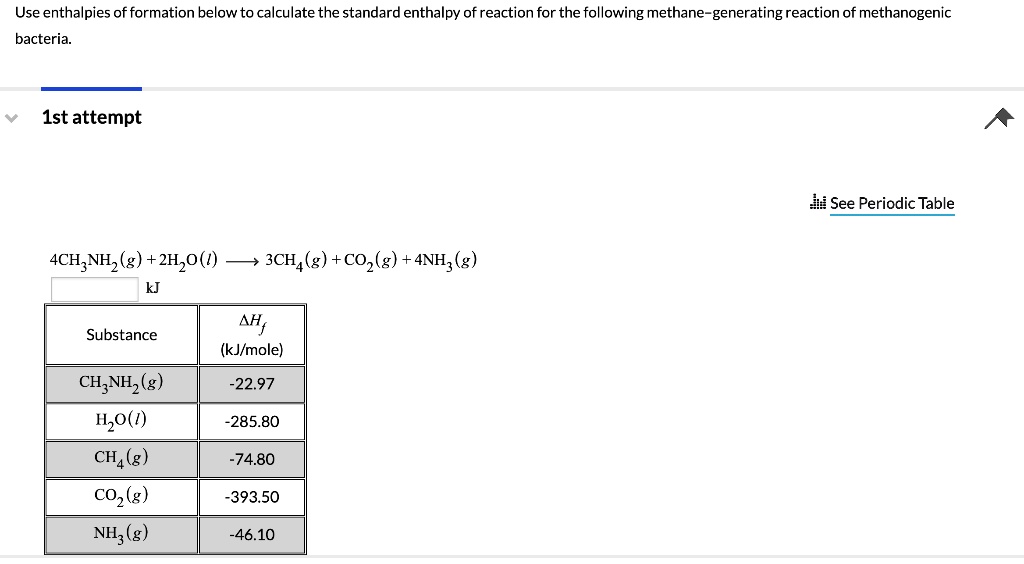
SOLVEDUse enthalpies of formation below to calculate the standard enthalpy of reaction for the
Standard Enthalpies Of Formation To Calculate Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure.
From www.chegg.com
Solved Use enthalpies of formation to calculate the standard Standard Enthalpies Of Formation To Calculate a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change. Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 H2O (g) Calculate the Standard Enthalpies Of Formation To Calculate 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
SOLVED Using the standard molar enthalpies of formation given, calculate the standard enthalpy Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of.. Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Appendix I to calculate the Standard Enthalpies Of Formation To Calculate 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of formation, δh^\circ_\ce {f},. Standard Enthalpies Of Formation To Calculate.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. to calculate the standard enthalpy of. Standard Enthalpies Of Formation To Calculate.
From studylib.net
Using Standard Molar Enthalpies of Formation to Calculate Enthalpy Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Enthalpies Of Formation To Calculate.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy... Course Hero Standard Enthalpies Of Formation To Calculate 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when. Standard Enthalpies Of Formation To Calculate.
From exoxhphey.blob.core.windows.net
Standard Enthalpy Of Formation Calculator at Susan blog Standard Enthalpies Of Formation To Calculate a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the. Standard Enthalpies Of Formation To Calculate.
From dxorvtvqp.blob.core.windows.net
Standard Enthalpy Of Formation Gibbsite at Samuel Speed blog Standard Enthalpies Of Formation To Calculate 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1. a. Standard Enthalpies Of Formation To Calculate.
From solvedlib.com
Using the standard molar enthalpies of formation give… SolvedLib Standard Enthalpies Of Formation To Calculate to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change. Standard Enthalpies Of Formation To Calculate.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpies Of Formation To Calculate Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change. Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
SOLVED Calculate the heats of combustion for the following reactions from the standard Standard Enthalpies Of Formation To Calculate 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created.. Standard Enthalpies Of Formation To Calculate.
From www.chegg.com
Solved Use standard enthalpies of formation to calculate Standard Enthalpies Of Formation To Calculate 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. to calculate the standard enthalpy of formation. Standard Enthalpies Of Formation To Calculate.
From www.chegg.com
Solved The standard enthalpies of formation for several Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1. Use. Standard Enthalpies Of Formation To Calculate.
From www.chegg.com
Solved Calculate the enthalpy of the following reaction Standard Enthalpies Of Formation To Calculate to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii). Standard Enthalpies Of Formation To Calculate.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpies Of Formation To Calculate to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation. Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
SOLVED Calculate the standard enthalpy of combustion for propane gas in kj/mol. the standard Standard Enthalpies Of Formation To Calculate to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly. Standard Enthalpies Of Formation To Calculate.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Change YouTube Standard Enthalpies Of Formation To Calculate to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. . Standard Enthalpies Of Formation To Calculate.
From www.youtube.com
How to Calculate the Standard Enthalpy of a Reaction from the Standard Enthalpies of Formation Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. a. Standard Enthalpies Of Formation To Calculate.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation, δh o f,. Standard Enthalpies Of Formation To Calculate.
From nasirghopbuck.blogspot.com
How to Calculate the Standard Enthalpy of Combustion Standard Enthalpies Of Formation To Calculate 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1. . Standard Enthalpies Of Formation To Calculate.
From kunduz.com
[ANSWERED] Use the standard enthalpies of formation to calculate the AH Kunduz Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1.. Standard Enthalpies Of Formation To Calculate.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpies Of Formation To Calculate Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of.. Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
SOLVEDUse standard enthalpies of formation in Appendix L to calculate enthalpy changes for the Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of. Standard Enthalpies Of Formation To Calculate.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube Standard Enthalpies Of Formation To Calculate Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance. Standard Enthalpies Of Formation To Calculate.
From www.chegg.com
Solved 15. Using standard enthalpies of formation, calculate Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. to calculate the standard enthalpy of formation of a compound, we must start with the elements in their.. Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of formation and standard enthalpy Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for. Standard Enthalpies Of Formation To Calculate.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpies Of Formation To Calculate a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, δh o f, is the enthalpy change. Standard Enthalpies Of Formation To Calculate.
From www.solutionspile.com
[Solved] Use standard enthalpies of formation to calculate Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. the standard enthalpy of formation, δh^\circ_\ce. Standard Enthalpies Of Formation To Calculate.
From www.bartleby.com
Answered Using the standard enthalpies of… bartleby Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Use standard enthalpies. Standard Enthalpies Of Formation To Calculate.
From www.youtube.com
Calculating Reaction Enthalpy from Enthalpies of Formation YouTube Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying. Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
SOLVEDUse enthalpies of formation below to calculate the standard enthalpy of reaction for the Standard Enthalpies Of Formation To Calculate Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change. Standard Enthalpies Of Formation To Calculate.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation, free download ID2964088 Standard Enthalpies Of Formation To Calculate 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their. Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
SOLVEDUse the standard enthalpies of formation available here to calculate the standard Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1. Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
SOLVED Calculate values for the standard reaction enthalpies at 298k for the reactions in Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. to calculate the standard enthalpy of formation of a compound, we must start with the elements in their. the standard enthalpy of formation, δh^\circ_\ce {f}, is the enthalpy change accompanying the formation of 1.. Standard Enthalpies Of Formation To Calculate.