What Temperature Does Air Burn . Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. That releases more energy, which releases more atoms. — for all these values, the initial. — adiabatic flame temperature is the temperature of complete combustion with no heat loss or gain to the environment. — fires burn only when all that atomic shuffling releases enough energy to keep the oxidation going in a sustained chain reaction. magnesium burns brightly in air and is used in distress flares. as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. This heats the oxygen — and so on. Some metal oxides dissolve in. This is a chart of adiabatic flame temperatures for common fuels. For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. More atoms released from the fuel combine with nearby oxygen.
from brandonkss.github.io
magnesium burns brightly in air and is used in distress flares. Some metal oxides dissolve in. This is a chart of adiabatic flame temperatures for common fuels. For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. — fires burn only when all that atomic shuffling releases enough energy to keep the oxidation going in a sustained chain reaction. Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. This heats the oxygen — and so on. More atoms released from the fuel combine with nearby oxygen. — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c.
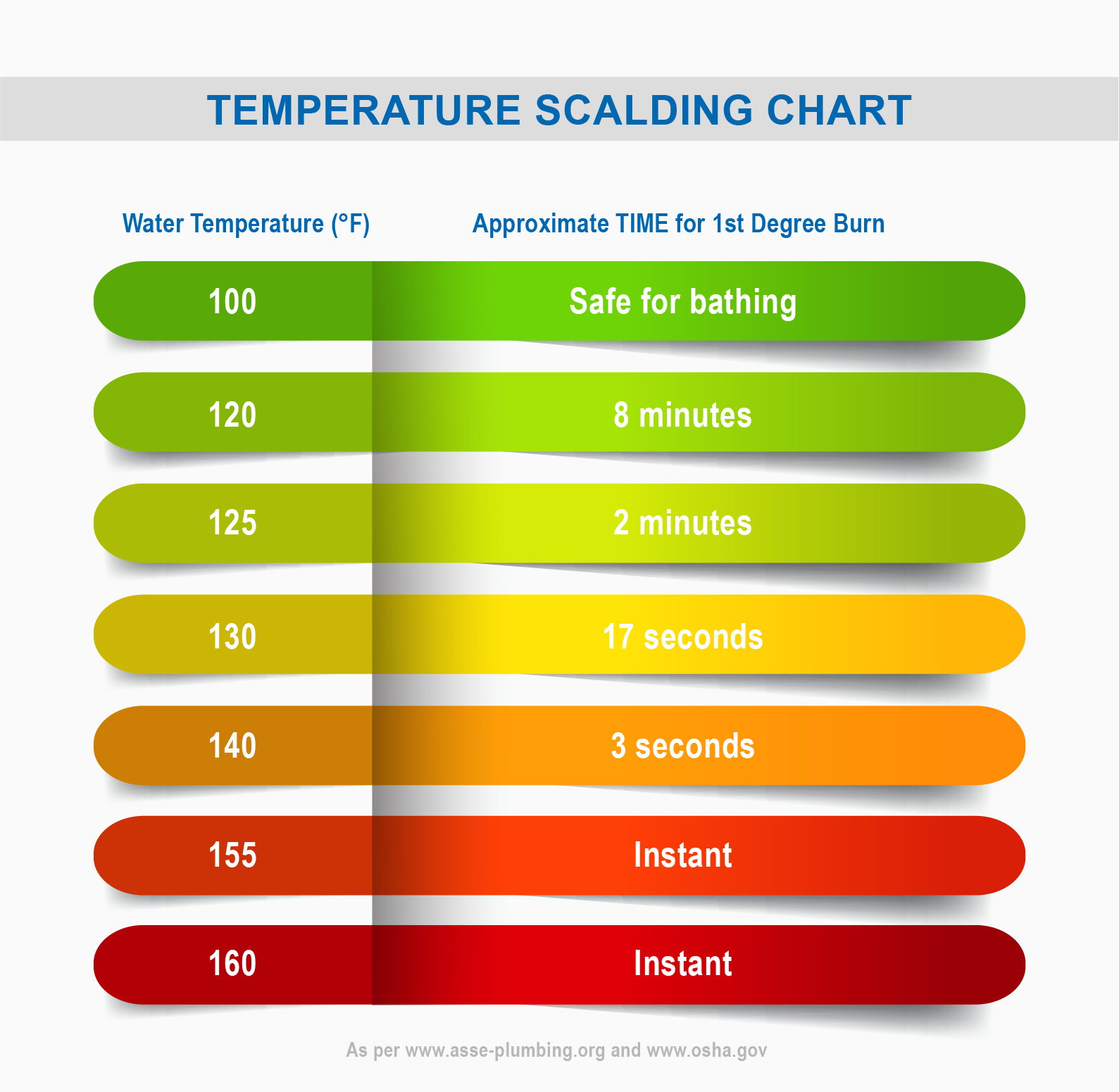
Skin Burn Temperature Chart
What Temperature Does Air Burn For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. — fires burn only when all that atomic shuffling releases enough energy to keep the oxidation going in a sustained chain reaction. Some metal oxides dissolve in. This heats the oxygen — and so on. — adiabatic flame temperature is the temperature of complete combustion with no heat loss or gain to the environment. — for all these values, the initial. as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. This is a chart of adiabatic flame temperatures for common fuels. magnesium burns brightly in air and is used in distress flares. That releases more energy, which releases more atoms. More atoms released from the fuel combine with nearby oxygen.
From yakimafire.com
Warm Weather Safety Yakima Fire Department What Temperature Does Air Burn — for all these values, the initial. This is a chart of adiabatic flame temperatures for common fuels. magnesium burns brightly in air and is used in distress flares. — adiabatic flame temperature is the temperature of complete combustion with no heat loss or gain to the environment. That releases more energy, which releases more atoms. . What Temperature Does Air Burn.
From mtmedgr.com
Burn Treatment Bend's First and Finest What Temperature Does Air Burn Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. — for all these values, the initial. For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. This heats the oxygen — and so on. magnesium burns brightly in air and is used in distress flares. This is a chart of. What Temperature Does Air Burn.
From southwestwoundcare.com
Acute Thermal Burn Injury Symptoms, Treatment in Lubbock Texas What Temperature Does Air Burn — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. This heats the oxygen — and so on. as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. — fires burn only when all that atomic shuffling releases enough energy to. What Temperature Does Air Burn.
From gardeningaid.com
What's the perfect temperature of burning THC? What Temperature Does Air Burn — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. This heats the oxygen — and so on. Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. This is a chart of adiabatic flame temperatures for common fuels. For most fuels, it’s around 2000 degrees celsius or. What Temperature Does Air Burn.
From learntoflyblog.com
Weather Temperature and Atmosphere Learn to Fly Blog ASA (Aviation What Temperature Does Air Burn This heats the oxygen — and so on. This is a chart of adiabatic flame temperatures for common fuels. magnesium burns brightly in air and is used in distress flares. More atoms released from the fuel combine with nearby oxygen. Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. Some metal oxides dissolve. What Temperature Does Air Burn.
From www.youtube.com
Does fire need air to burn Science Project Class 6 YouTube What Temperature Does Air Burn — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. This heats the oxygen — and so on. as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. magnesium burns brightly in air and is used in distress flares. —. What Temperature Does Air Burn.
From huntingwaterfalls.com
What Temperature Does Butane Burn At? What Temperature Does Air Burn — adiabatic flame temperature is the temperature of complete combustion with no heat loss or gain to the environment. This heats the oxygen — and so on. — fires burn only when all that atomic shuffling releases enough energy to keep the oxidation going in a sustained chain reaction. Metal oxides produced in oxidation reactions are bases, they. What Temperature Does Air Burn.
From dxoyufult.blob.core.windows.net
At What Point Does Humidity Make It Feel Colder at Sean Eubanks blog What Temperature Does Air Burn For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. — adiabatic flame temperature is the temperature of complete combustion with no heat loss or gain to the environment. — for all these values, the initial. This is a chart of adiabatic flame temperatures for common fuels. magnesium burns brightly in air and is used. What Temperature Does Air Burn.
From dxovpsuca.blob.core.windows.net
How Cold Should Hvac Air Be at Emily Cook blog What Temperature Does Air Burn That releases more energy, which releases more atoms. — fires burn only when all that atomic shuffling releases enough energy to keep the oxidation going in a sustained chain reaction. — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. — for all these values, the initial. For most fuels, it’s. What Temperature Does Air Burn.
From firstaidmart.com
burn care / First Aid Mart Official Blog What Temperature Does Air Burn Some metal oxides dissolve in. magnesium burns brightly in air and is used in distress flares. — adiabatic flame temperature is the temperature of complete combustion with no heat loss or gain to the environment. This heats the oxygen — and so on. Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them.. What Temperature Does Air Burn.
From www.pieringlawfirm.com
What Are the Different Degrees of Burn Injuries? Piering Law Firm What Temperature Does Air Burn as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. Some metal oxides dissolve in. — for all these values, the initial. This heats the oxygen — and so on. For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. That releases more energy, which releases. What Temperature Does Air Burn.
From sciencenotes.org
Adiabatic Flame Temperature Chart What Temperature Does Air Burn Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. magnesium burns brightly in air and is used in distress flares. This heats the oxygen — and so on. — for all these values, the initial. — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c.. What Temperature Does Air Burn.
From reliablewater247.com
Benefits of Booster Heaters Reliable Water Services What Temperature Does Air Burn — fires burn only when all that atomic shuffling releases enough energy to keep the oxidation going in a sustained chain reaction. as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. This heats the oxygen — and so on. — adiabatic flame temperature is the temperature. What Temperature Does Air Burn.
From www.fluorined-chemicals.com
What Temperature Does Propane Burn At News Xiamen Juda Chemical What Temperature Does Air Burn — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. That releases more energy, which releases more atoms. This is a chart of adiabatic flame temperatures for common fuels. Some metal oxides dissolve in. — for all these values, the initial. magnesium burns brightly in air and is used in distress. What Temperature Does Air Burn.
From www.youtube.com
At what temperature does condensation occur? YouTube What Temperature Does Air Burn For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. This heats the oxygen — and so on. More atoms released from the fuel combine with nearby oxygen. Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. — fires burn only when all that atomic shuffling releases enough energy to keep. What Temperature Does Air Burn.
From brandonkss.github.io
Skin Burn Temperature Chart What Temperature Does Air Burn This heats the oxygen — and so on. — fires burn only when all that atomic shuffling releases enough energy to keep the oxidation going in a sustained chain reaction. magnesium burns brightly in air and is used in distress flares. — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c.. What Temperature Does Air Burn.
From www.cityfire.co.uk
The Temperature Of Fire City Fire Protection What Temperature Does Air Burn Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. That releases more energy, which releases more atoms. as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. — adiabatic flame temperature is the temperature of complete combustion with no heat loss or. What Temperature Does Air Burn.
From brandonkss.github.io
Skin Burn Temperature Chart What Temperature Does Air Burn as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. Some metal oxides dissolve in. magnesium burns brightly in air and is used in distress flares. This heats the oxygen — and so on. — for all these values, the initial. For most fuels, it’s around 2000. What Temperature Does Air Burn.
From www.grc.nasa.gov
Combustion What Temperature Does Air Burn For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. Some metal oxides dissolve in. More atoms released from the fuel combine with nearby oxygen. — adiabatic flame temperature is the temperature of complete combustion with no heat loss or gain to the environment. — conventional domestic flames for heat and light tend to reach between. What Temperature Does Air Burn.
From www.firstaidforfree.com
What Are The Different Types of Burn Injuries? What Temperature Does Air Burn — adiabatic flame temperature is the temperature of complete combustion with no heat loss or gain to the environment. This is a chart of adiabatic flame temperatures for common fuels. Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. More atoms released from the fuel combine with nearby oxygen. Some metal oxides dissolve. What Temperature Does Air Burn.
From www.kidsafesa.com.au
Burn temperature Kidsafe SA What Temperature Does Air Burn That releases more energy, which releases more atoms. This heats the oxygen — and so on. Some metal oxides dissolve in. For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. Metal oxides produced in oxidation reactions are bases, they react. What Temperature Does Air Burn.
From share.upmc.com
Burn Treatment and Degrees of Burn UPMC HealthBeat What Temperature Does Air Burn Some metal oxides dissolve in. — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. — fires burn only when all that atomic shuffling releases enough energy to keep the oxidation going in a sustained chain reaction. For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. as air. What Temperature Does Air Burn.
From www.chemtrailplanet.com
Temperatures at which different materials burn or melt What Temperature Does Air Burn — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. Some metal oxides dissolve in. That releases more energy, which releases more atoms. as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. — fires burn only when all that atomic. What Temperature Does Air Burn.
From www.verywellhealth.com
How Different Degrees of Burns Are Treated What Temperature Does Air Burn — adiabatic flame temperature is the temperature of complete combustion with no heat loss or gain to the environment. For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. Metal oxides produced in oxidation reactions are. What Temperature Does Air Burn.
From www.youtube.com
Burn Part 1 Types, Degrees and Classification YouTube What Temperature Does Air Burn — adiabatic flame temperature is the temperature of complete combustion with no heat loss or gain to the environment. That releases more energy, which releases more atoms. — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. Metal oxides produced. What Temperature Does Air Burn.
From www.jbsa.mil
Know the difference between a scald and burn > Joint Base San Antonio What Temperature Does Air Burn This is a chart of adiabatic flame temperatures for common fuels. More atoms released from the fuel combine with nearby oxygen. as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. . What Temperature Does Air Burn.
From mammothmemory.net
When metals are heated it reacts with oxygen to create flame What Temperature Does Air Burn For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. This heats the oxygen — and so on. Some metal oxides dissolve in. magnesium burns brightly in air and is used in distress flares. — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. — adiabatic flame temperature is. What Temperature Does Air Burn.
From sciencenotes.org
Why Is Fire Hot? How Hot Is It? What Temperature Does Air Burn — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. — for all these values, the initial. This is a chart of adiabatic flame temperatures for common fuels. — adiabatic flame temperature is the temperature of. What Temperature Does Air Burn.
From www.doubtnut.com
Propane burns in air according to the following equation C3H8 +5O2 What Temperature Does Air Burn This is a chart of adiabatic flame temperatures for common fuels. Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. — fires burn only when all that atomic shuffling releases enough energy to keep the oxidation going in a sustained chain reaction. For most fuels, it’s around 2000 degrees celsius or 3500 degrees. What Temperature Does Air Burn.
From brandonkss.github.io
Skin Burn Temperature Chart What Temperature Does Air Burn — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. — for all these values, the initial. magnesium burns brightly in air and is used in distress flares. For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. Some metal oxides dissolve in. This heats the oxygen — and. What Temperature Does Air Burn.
From firesafeliving.com
What Temperature Does Paper Burn? Explained What Temperature Does Air Burn More atoms released from the fuel combine with nearby oxygen. Some metal oxides dissolve in. as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. — for all these values, the initial. — fires burn only when all that atomic shuffling releases enough energy to keep the. What Temperature Does Air Burn.
From ektalks.blogspot.com
ektalks Why Does Steam Cause More Severe Burns Than Hot Water What Temperature Does Air Burn as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. — for all these values, the initial. This heats the oxygen — and so on. More atoms released from the fuel combine with nearby oxygen. magnesium burns brightly in air and is used in distress flares. . What Temperature Does Air Burn.
From www.slideserve.com
PPT How does temperature affect the rate at which a candle burns What Temperature Does Air Burn — for all these values, the initial. — conventional domestic flames for heat and light tend to reach between about 800°c and 1000°c. as air heats there are compounds that will favorably form from oxygen and nitrogen that don't form with regular temperature. That releases more energy, which releases more atoms. For most fuels, it’s around 2000. What Temperature Does Air Burn.
From unifirstfirstaidandsafety.com
Four Critical Steps To Burn Treatment When Burns Occur What Temperature Does Air Burn More atoms released from the fuel combine with nearby oxygen. — adiabatic flame temperature is the temperature of complete combustion with no heat loss or gain to the environment. That releases more energy, which releases more atoms. This is a chart of adiabatic flame temperatures for common fuels. as air heats there are compounds that will favorably form. What Temperature Does Air Burn.
From www.vectorstock.com
Medical burn stages degree burns Royalty Free Vector Image What Temperature Does Air Burn — fires burn only when all that atomic shuffling releases enough energy to keep the oxidation going in a sustained chain reaction. magnesium burns brightly in air and is used in distress flares. For most fuels, it’s around 2000 degrees celsius or 3500 degrees fahrenheit. This heats the oxygen — and so on. — adiabatic flame temperature. What Temperature Does Air Burn.