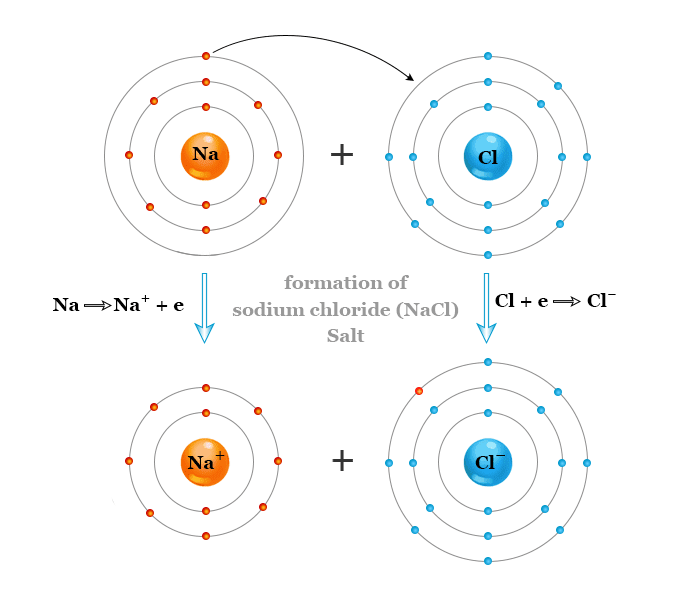
Chloride Density Formula . Sodium chloride is the chemical name of nacl. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. Visit byju's to understand the. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Its dissolution in water is endothermic, which means it takes some heat energy away from the water. The nacl molecular weight (sodium chloride) is 58.44 g/mol. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. The chemical formula of sodium chloride is nacl. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The molecular formula of table salt—sodium chloride—is nacl.
from www.priyamstudycentre.com
This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The molecular formula of table salt—sodium chloride—is nacl. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. Sodium chloride is the chemical name of nacl. Visit byju's to understand the. Its dissolution in water is endothermic, which means it takes some heat energy away from the water. The nacl molecular weight (sodium chloride) is 58.44 g/mol. The chemical formula of sodium chloride is nacl. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge.
Sodium Chloride (NaCl) Uses, Crystal
Chloride Density Formula The molecular formula of table salt—sodium chloride—is nacl. The molecular formula of table salt—sodium chloride—is nacl. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The chemical formula of sodium chloride is nacl. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Visit byju's to understand the. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. Sodium chloride is the chemical name of nacl. Its dissolution in water is endothermic, which means it takes some heat energy away from the water. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. The nacl molecular weight (sodium chloride) is 58.44 g/mol.
From www.nagwa.com
Question Video Understanding How Electron Density Is Distributed in Chloride Density Formula Visit byju's to understand the. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. Its dissolution in water is endothermic, which means it takes some heat energy away from the water. Explain why the boiling points of alcohols and. Chloride Density Formula.
From chemistry291.blogspot.com
Chemical Formula for Sodium ChlorideSodium Chloride Formula Chloride Density Formula The chemical formula of sodium chloride is nacl. Sodium chloride is the chemical name of nacl. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. The nacl molecular weight (sodium chloride) is 58.44 g/mol. In the solid lattice, each. Chloride Density Formula.
From www.toppr.com
The pycnometric density of sodium chloride crystal is 2.165 × 10^3 kg m Chloride Density Formula This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Sodium chloride is the chemical name of nacl. The nacl molecular weight (sodium chloride) is 58.44. Chloride Density Formula.
From www.numerade.com
Find the percent by mass of sodium chloride in a Chloride Density Formula The molecular formula of table salt—sodium chloride—is nacl. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. The chemical formula of sodium chloride is nacl. Explain why the boiling points of alcohols and phenols are much higher than those. Chloride Density Formula.
From learnaboutpharma.com
Density Definition, Units, Calculations and Explanation Chloride Density Formula The chemical formula of sodium chloride is nacl. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. Sodium chloride is the chemical name of nacl. The nacl molecular weight (sodium chloride) is 58.44 g/mol. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of. Chloride Density Formula.
From www.researchgate.net
Simulated and observed breakthrough curves of the chloride Chloride Density Formula The molecular formula of table salt—sodium chloride—is nacl. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Sodium chloride is the chemical name of nacl. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,.. Chloride Density Formula.
From stock.adobe.com
Sodium chloride (table salt), chemical structure. Skeletal formula Chloride Density Formula In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. The chemical formula of sodium chloride is nacl. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. Explain why the boiling points of alcohols and phenols are much higher than those. Chloride Density Formula.
From www.meritnation.com
The density of crystalline sodium chloride is 5 85gcm3 What is the Chloride Density Formula Its dissolution in water is endothermic, which means it takes some heat energy away from the water. The chemical formula of sodium chloride is nacl. Visit byju's to understand the. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. The molecular formula of table salt—sodium chloride—is nacl. This formula indicates that each unit. Chloride Density Formula.
From brainly.in
The equivalent weight of an element is 4 it's chloride has vapour Chloride Density Formula The chemical formula of sodium chloride is nacl. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Sodium chloride is the chemical name of nacl. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing. Chloride Density Formula.
From www.toppr.com
If the unit cell length of sodium chloride crystal is 600pm, then its Chloride Density Formula In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. Visit byju's to understand the. The nacl molecular weight (sodium chloride) is 58.44 g/mol. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. The chemical formula of sodium chloride is nacl.. Chloride Density Formula.
From www.numerade.com
SOLVED 'A modified Ringers Irrigation has the following formula Chloride Density Formula Visit byju's to understand the. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The molecular formula of table salt—sodium chloride—is nacl. Sodium chloride is the chemical name of nacl. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with. Chloride Density Formula.
From www.numerade.com
The crystal structure of sodium chloride (NaCl) is a simple cubic with Chloride Density Formula The chemical formula of sodium chloride is nacl. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. The molecular formula of table salt—sodium chloride—is nacl. In the solid lattice, each ion is surrounded by six ions having an opposite. Chloride Density Formula.
From www.doubtnut.com
The pyknometric density of sodium chloride crystal is 2.165xx10^(3)kg Chloride Density Formula The nacl molecular weight (sodium chloride) is 58.44 g/mol. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. Sodium chloride is the chemical name of nacl. The chemical formula of sodium chloride is nacl. The molecular formula of table salt—sodium chloride—is nacl. This formula indicates that each unit of sodium chloride is composed. Chloride Density Formula.
From www.chegg.com
Solved what is the density of 22 NaCl solution at 50 Chloride Density Formula Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. The molecular formula of table salt—sodium chloride—is nacl. Its dissolution in water is endothermic, which means it takes some heat energy away from the water. Visit byju's to understand the.. Chloride Density Formula.
From questions-in.kunduz.com
84. (1) 0.3 M (2) 0.2 M (3) 0.6 M (4) 1.8... Physical Chemistry Chloride Density Formula This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. Its dissolution in water is endothermic, which means it takes some heat energy away from the water. Sodium chloride is the chemical name of nacl. In the solid lattice, each ion is surrounded by six ions having an. Chloride Density Formula.
From byjus.com
A solid element form gaseous chloride on reaction with cl2(at certain Chloride Density Formula Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Its dissolution in water is endothermic, which means. Chloride Density Formula.
From kunduz.com
[ANSWERED] The pyknometric density of sodium chloride crystal is 2 165 Chloride Density Formula Sodium chloride is the chemical name of nacl. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The chemical formula of sodium chloride is nacl. Its dissolution in water is endothermic, which means it takes some heat energy away from the water. The nacl molecular weight (sodium. Chloride Density Formula.
From www.researchgate.net
9. Density of lithium chloridewater Download Scientific Diagram Chloride Density Formula This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The molecular formula of table salt—sodium chloride—is nacl. Sodium chloride is the chemical name of nacl. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing. Chloride Density Formula.
From www.engineeringtoolbox.com
Density of aqueous solutions of chlorides Chloride Density Formula Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. The chemical formula of sodium chloride is nacl. The nacl molecular weight (sodium chloride) is 58.44 g/mol. Visit byju's to understand the. In the solid lattice, each ion is surrounded. Chloride Density Formula.
From www.numerade.com
SOLVEDExcess sodium is reacted with 5.00 Lof chlorine gas that has Chloride Density Formula In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. The chemical formula of sodium chloride is nacl. The molecular formula of table salt—sodium chloride—is nacl. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. Sodium chloride is the chemical name. Chloride Density Formula.
From www.numerade.com
SOLVED Question 9 solid sodium chloride is produced by the reaction of Chloride Density Formula Sodium chloride is the chemical name of nacl. The chemical formula of sodium chloride is nacl. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. The nacl molecular weight (sodium chloride) is 58.44 g/mol. Sodium chloride, also known as salt, common salt, table salt or halite, is. Chloride Density Formula.
From encyclopedia.airliquide.com
Hydrogen chloride Gas Encyclopedia Air Liquide Chloride Density Formula Visit byju's to understand the. The chemical formula of sodium chloride is nacl. The molecular formula of table salt—sodium chloride—is nacl. Sodium chloride is the chemical name of nacl. The nacl molecular weight (sodium chloride) is 58.44 g/mol. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. Its dissolution in water is endothermic,. Chloride Density Formula.
From www.engineeringtoolbox.com
Calcium Chloride and Water Chloride Density Formula Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. Its dissolution in water is endothermic, which means it takes some heat energy away from the water. The molecular formula of table salt—sodium chloride—is nacl. Visit byju's to understand the.. Chloride Density Formula.
From www.numerade.com
SOLVEDConsider an aqueous solution containing sodium chloride that has Chloride Density Formula This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The molecular formula of table salt—sodium chloride—is nacl. Sodium chloride is the chemical name of nacl. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass.. Chloride Density Formula.
From www.numerade.com
A chloride of silicon contains 79.1 mass Cl. (a) What is the Chloride Density Formula Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The nacl molecular weight (sodium chloride) is 58.44 g/mol. Visit byju's to understand the. Sodium chloride,. Chloride Density Formula.
From studylib.net
DENSITY OF AQUEOUS SODIUM CHLORIDE SOLUTIONS Experiment 3 Chloride Density Formula Its dissolution in water is endothermic, which means it takes some heat energy away from the water. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. The chemical formula of sodium chloride is nacl. Visit byju's to understand the. The molecular formula of table salt—sodium chloride—is nacl. The nacl molecular weight (sodium chloride). Chloride Density Formula.
From www.toppr.com
A unit cell of sodium chloride has four formula units. The edge length Chloride Density Formula The molecular formula of table salt—sodium chloride—is nacl. The chemical formula of sodium chloride is nacl. Sodium chloride is the chemical name of nacl. Its dissolution in water is endothermic, which means it takes some heat energy away from the water. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride. Chloride Density Formula.
From www.chegg.com
Solved L. Density of aqueous sodium chloride (NaC) solution Chloride Density Formula The chemical formula of sodium chloride is nacl. The nacl molecular weight (sodium chloride) is 58.44 g/mol. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. Visit byju's to understand the. The molecular formula of table salt—sodium chloride—is nacl. Explain why the boiling points of alcohols and. Chloride Density Formula.
From www.researchgate.net
Density of vinyl chloride as a function of pressure (a) and deviation Chloride Density Formula In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. Its dissolution in water is endothermic, which means it takes some heat energy away from the water. The chemical formula of sodium chloride is nacl. The molecular formula of table salt—sodium chloride—is nacl. Explain why the boiling points of alcohols and phenols are much. Chloride Density Formula.
From asideload7.gitlab.io
Formidable Word Equation Of Sodium Chloride Edexcel Physics Syllabus Chloride Density Formula Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. The nacl molecular weight (sodium chloride) is 58.44. Chloride Density Formula.
From byjus.com
A unit cell of sodium chloride has four formula units.The edge length Chloride Density Formula Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Its dissolution in water is endothermic, which means it takes some heat energy away from the water. The molecular formula of table salt—sodium chloride—is nacl. This formula indicates that each unit of sodium chloride is composed of one. Chloride Density Formula.
From www.priyamstudycentre.com
Sodium Chloride (NaCl) Uses, Crystal Chloride Density Formula This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. Its dissolution in water is endothermic, which means it takes some heat energy away from the water. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular. Chloride Density Formula.
From edurev.in
Vapour density of a metal chloride is 66 . its oxide contains 53 metal Chloride Density Formula Visit byju's to understand the. This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The molecular formula of table salt—sodium chloride—is nacl. The nacl molecular weight (sodium chloride) is 58.44 g/mol. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound. Chloride Density Formula.
From www.youtube.com
Calculating MOLALITY using DENSITY! YouTube Chloride Density Formula This formula indicates that each unit of sodium chloride is composed of one sodium ion (na⁺) and one chloride ion (cl⁻) ,. The nacl molecular weight (sodium chloride) is 58.44 g/mol. In the solid lattice, each ion is surrounded by six ions having an opposite electrical charge. The molecular formula of table salt—sodium chloride—is nacl. Sodium chloride is the chemical. Chloride Density Formula.
From www.toppr.com
The pycnometric density of sodium chloride crystal is 2.165 × 10^3 kg m Chloride Density Formula Its dissolution in water is endothermic, which means it takes some heat energy away from the water. The molecular formula of table salt—sodium chloride—is nacl. The chemical formula of sodium chloride is nacl. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Visit byju's to understand the.. Chloride Density Formula.