Common Salt Laboratory Preparation . See examples, reactions, and definitions of salts and their ions. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms. They can then recover this salt by crystallisation. Find out its properties, structure and uses in various industries and applications. Preparation of simple inorganic salt solutions. Preparations of acid and base solutions. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. Basic concepts of preparing solutions. An acid and an alkali react to form a soluble salt in solution. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. Access free videos to support your teaching.
from www.dreamstime.com
Preparations of acid and base solutions. Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms. Find out its properties, structure and uses in various industries and applications. Access free videos to support your teaching. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. Basic concepts of preparing solutions. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. See examples, reactions, and definitions of salts and their ions. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. They can then recover this salt by crystallisation.
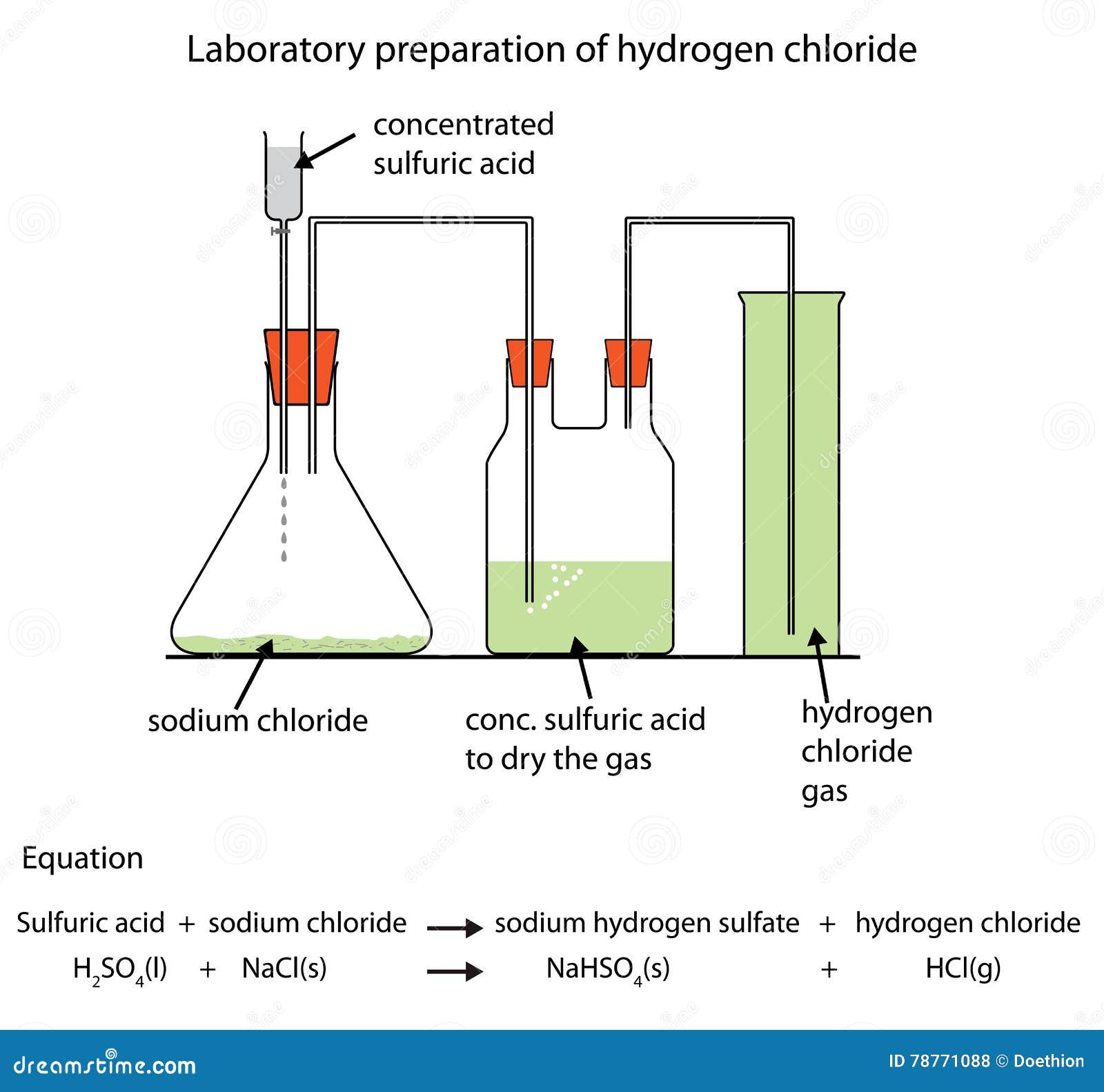
Diagram of Preparation of Hydrogen Chloride Gas Stock Illustration
Common Salt Laboratory Preparation In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. Preparations of acid and base solutions. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. They can then recover this salt by crystallisation. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. Find out its properties, structure and uses in various industries and applications. Access free videos to support your teaching. Basic concepts of preparing solutions. See examples, reactions, and definitions of salts and their ions. An acid and an alkali react to form a soluble salt in solution. Preparation of simple inorganic salt solutions. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms.
From chemnotcheem.com
Preparation of salts O Level Chemistry Notes Common Salt Laboratory Preparation Preparations of acid and base solutions. Find out its properties, structure and uses in various industries and applications. Basic concepts of preparing solutions. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. See examples, reactions, and definitions of salts and their ions. An acid and an alkali. Common Salt Laboratory Preparation.
From www.dreamstime.com
Diagram of Preparation of Hydrogen Chloride Gas Stock Illustration Common Salt Laboratory Preparation Preparations of acid and base solutions. Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms. See examples, reactions, and definitions of salts and their ions. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. An acid and an alkali react to form a soluble. Common Salt Laboratory Preparation.
From www.slideshare.net
Salt preparation by titration Common Salt Laboratory Preparation Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms. Basic concepts of preparing solutions. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. See examples, reactions, and definitions of. Common Salt Laboratory Preparation.
From rekhasharma22.blogspot.com
apsg chemistry practical for claa9 Common Salt Laboratory Preparation Find out its properties, structure and uses in various industries and applications. See examples, reactions, and definitions of salts and their ions. Basic concepts of preparing solutions. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation. Common Salt Laboratory Preparation.
From www.youtube.com
Experiment Preparation of soluble salt by titration YouTube Common Salt Laboratory Preparation In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. They can then recover this salt by crystallisation. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses.. Common Salt Laboratory Preparation.
From sajhanotes.com
Separation of Sand and Common Salt Chemistry Lab Sajha Notes Common Salt Laboratory Preparation Access free videos to support your teaching. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. They can then recover this salt by crystallisation. Preparations of acid and base solutions. An acid and an alkali react to form a soluble salt in solution. Basic concepts of preparing solutions. Learn how sodium chloride (nacl) is. Common Salt Laboratory Preparation.
From www.vedantu.com
Chemicals From Common Salt Learn Important Terms and Concepts Common Salt Laboratory Preparation An acid and an alkali react to form a soluble salt in solution. Find out its properties, structure and uses in various industries and applications. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. Preparations of acid and base solutions. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric. Common Salt Laboratory Preparation.
From docslib.org
Lab Session 4, Experiment 3 Preparation of Sodium Chloride DocsLib Common Salt Laboratory Preparation Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. Find out its properties, structure and uses in various industries and applications. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. Learn how sodium chloride, also known as table salt or common. Common Salt Laboratory Preparation.
From www.youtube.com
Preparation of Salts YouTube Common Salt Laboratory Preparation See examples, reactions, and definitions of salts and their ions. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. Basic concepts of preparing solutions. Learn how sodium chloride, also known as table. Common Salt Laboratory Preparation.
From www.slideserve.com
PPT Preparation of Salts PowerPoint Presentation, free download ID Common Salt Laboratory Preparation In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. See examples, reactions, and definitions of salts and their ions. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. Find out its properties, structure and uses in various industries and applications. An. Common Salt Laboratory Preparation.
From sciphychem.blogspot.com
SciPhyChem (O level Chem) How to prepare salts Common Salt Laboratory Preparation Access free videos to support your teaching. See examples, reactions, and definitions of salts and their ions. Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms. An acid and an alkali react to form a soluble salt in solution. They can then recover this salt by crystallisation. Learn how sodium. Common Salt Laboratory Preparation.
From www.labkafe.com
Color of Common Salts Used in School Laboratories Labkafe Common Salt Laboratory Preparation In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. See examples, reactions, and definitions of salts and their ions. Find out its properties, structure and uses in various industries and applications. Access free videos to support your teaching. They. Common Salt Laboratory Preparation.
From www.achieversdream.com.sg
Achievers Dream 3 Methods for Salt Preparation Common Salt Laboratory Preparation Preparation of simple inorganic salt solutions. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. Access free videos to support your teaching. Find out its properties, structure and uses in various industries and applications. An acid and an alkali react to form a soluble salt in solution. Basic concepts of preparing solutions. They can. Common Salt Laboratory Preparation.
From www.slideshare.net
Salt preparation Common Salt Laboratory Preparation Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. Basic concepts of preparing solutions. Access free videos to support your teaching. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. Find out its properties, structure and uses in various industries and. Common Salt Laboratory Preparation.
From www.youtube.com
Making A true solution of common salt,sugar & alumClass9 Experiment Common Salt Laboratory Preparation Find out its properties, structure and uses in various industries and applications. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. Access free videos to support your teaching. Preparations of acid and base solutions. See examples, reactions, and definitions of salts and their ions. Preparation of simple. Common Salt Laboratory Preparation.
From www.slideserve.com
PPT Preparation of Salts PowerPoint Presentation, free download ID Common Salt Laboratory Preparation See examples, reactions, and definitions of salts and their ions. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. Preparations of acid and base solutions. Access free videos to support your teaching. They can then recover this salt by crystallisation. Preparation of simple inorganic salt solutions. In. Common Salt Laboratory Preparation.
From slidetodoc.com
CHAPTER 12 Salts 2013 Marshall Cavendish International Singapore Common Salt Laboratory Preparation Preparations of acid and base solutions. Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms. Access free videos to support your teaching. Basic concepts of preparing solutions. Preparation of simple inorganic salt solutions. See examples, reactions, and definitions of salts and their ions. In this experiment, students produce ammonium sulfate. Common Salt Laboratory Preparation.
From www.slideshare.net
8.1 (b) Preparation of Soluble salts Common Salt Laboratory Preparation In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. Access free videos to support your teaching. Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms. Preparations of acid and base solutions. An acid and an alkali react to form a soluble salt in solution.. Common Salt Laboratory Preparation.
From www.slideshare.net
8.1 (b) Preparation of Soluble salts Common Salt Laboratory Preparation In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. Preparations of acid and base solutions. Basic concepts of preparing solutions. See examples, reactions, and definitions of salts and their ions. Preparation of simple inorganic salt solutions. Access free videos to support your teaching. Learn how sodium chloride, also known as table salt or common. Common Salt Laboratory Preparation.
From www.aplustopper.com
Preparation of Salts A Plus Topper Common Salt Laboratory Preparation Preparations of acid and base solutions. They can then recover this salt by crystallisation. An acid and an alkali react to form a soluble salt in solution. Access free videos to support your teaching. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. In this experiment, students produce ammonium sulfate from the reaction between. Common Salt Laboratory Preparation.
From www.youtube.com
SCTS ChemCBSE 9th Is Matter around us Pure To prepare true Common Salt Laboratory Preparation Preparations of acid and base solutions. See examples, reactions, and definitions of salts and their ions. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. Access free videos to support your teaching. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses.. Common Salt Laboratory Preparation.
From studyrocket.co.uk
Preparation of Salts GCSE Chemistry Science) OCR Revision Common Salt Laboratory Preparation Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. An acid and an alkali react to form a soluble salt in solution. See examples, reactions, and definitions of salts and their ions. Access free videos to support your teaching. Learn how sodium chloride, also known as table. Common Salt Laboratory Preparation.
From www.youtube.com
Laboratory preparation of some salts part 1 YouTube Common Salt Laboratory Preparation In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. Basic concepts of preparing solutions. Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and. Common Salt Laboratory Preparation.
From www.nagwa.com
Question Video Explaining the Steps in the Preparation of a Soluble Common Salt Laboratory Preparation Find out its properties, structure and uses in various industries and applications. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. See examples, reactions, and definitions of salts and their ions. Basic concepts of preparing solutions. They can then recover this salt by crystallisation. An acid and an alkali react to form a soluble. Common Salt Laboratory Preparation.
From www.slideshare.net
Salt preparation by titration Common Salt Laboratory Preparation See examples, reactions, and definitions of salts and their ions. Basic concepts of preparing solutions. An acid and an alkali react to form a soluble salt in solution. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and. Common Salt Laboratory Preparation.
From www.youtube.com
To separate a mixture of Ammonium chloride (NH4Cl) and common salt Common Salt Laboratory Preparation Find out its properties, structure and uses in various industries and applications. See examples, reactions, and definitions of salts and their ions. Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. In this experiment, students. Common Salt Laboratory Preparation.
From narodnatribuna.info
Sodium Chloride Preparation Properties Structure Uses Common Salt Laboratory Preparation Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms. Access free videos to support your teaching. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. Preparation of simple inorganic salt solutions. They can then recover this salt by crystallisation. Basic concepts of preparing solutions.. Common Salt Laboratory Preparation.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Common Salt Laboratory Preparation Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. See examples, reactions, and definitions of salts and their ions. Preparations of acid and base solutions. Preparation of simple inorganic salt solutions. Access free videos to support your teaching. Learn how sodium chloride, also known as table salt. Common Salt Laboratory Preparation.
From www.slideserve.com
PPT Preparation of Salts PowerPoint Presentation, free download ID Common Salt Laboratory Preparation Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. An acid and an alkali react to form a soluble salt in solution. Preparation of simple inorganic salt solutions. Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms.. Common Salt Laboratory Preparation.
From www.youtube.com
Common salt reaction in NaOH preparation CBSE Class 10 Chemistry Notes Common Salt Laboratory Preparation Learn how sodium chloride, also known as table salt or common salt, is prepared from sodium and chlorine atoms. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. Preparations of acid and base solutions. They can then recover this salt by crystallisation. In laboratory preparation of salt,. Common Salt Laboratory Preparation.
From www.bbc.com
3. Investigate the preparation of soluble salts Revision 1 GCSE Common Salt Laboratory Preparation Basic concepts of preparing solutions. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. Preparations of acid and base solutions. An acid and an alkali react to form a soluble salt in solution. Preparation of simple inorganic salt solutions. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid.. Common Salt Laboratory Preparation.
From www.youtube.com
Class 10 Science Chapter 2 Acids Bases & Salts Some Common Salts Common Salt Laboratory Preparation An acid and an alkali react to form a soluble salt in solution. See examples, reactions, and definitions of salts and their ions. Preparations of acid and base solutions. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. They can then recover this salt by crystallisation. Find. Common Salt Laboratory Preparation.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Common Salt Laboratory Preparation See examples, reactions, and definitions of salts and their ions. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. An acid and an alkali react to form a soluble salt in solution. Access free videos to support your teaching. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid.. Common Salt Laboratory Preparation.
From www.oakabooks.co.uk
KS4/GCSE Making an Insoluble Salt Revision Resource For Dyslexics Common Salt Laboratory Preparation Preparation of simple inorganic salt solutions. Preparations of acid and base solutions. In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid. Learn how sodium chloride (nacl) is prepared by evaporation of seawater or mining of deposits, and what are its properties and uses. An acid and an alkali react to form a soluble salt. Common Salt Laboratory Preparation.
From www.youtube.com
Chemical Obtained from Common SaltI Class X (Chap 2 Part XIII) YouTube Common Salt Laboratory Preparation Find out its properties, structure and uses in various industries and applications. Preparation of simple inorganic salt solutions. Basic concepts of preparing solutions. See examples, reactions, and definitions of salts and their ions. Preparations of acid and base solutions. In laboratory preparation of salt, generally employed methods are neutralisation, precipitation reaction, the reaction between. Access free videos to support your. Common Salt Laboratory Preparation.