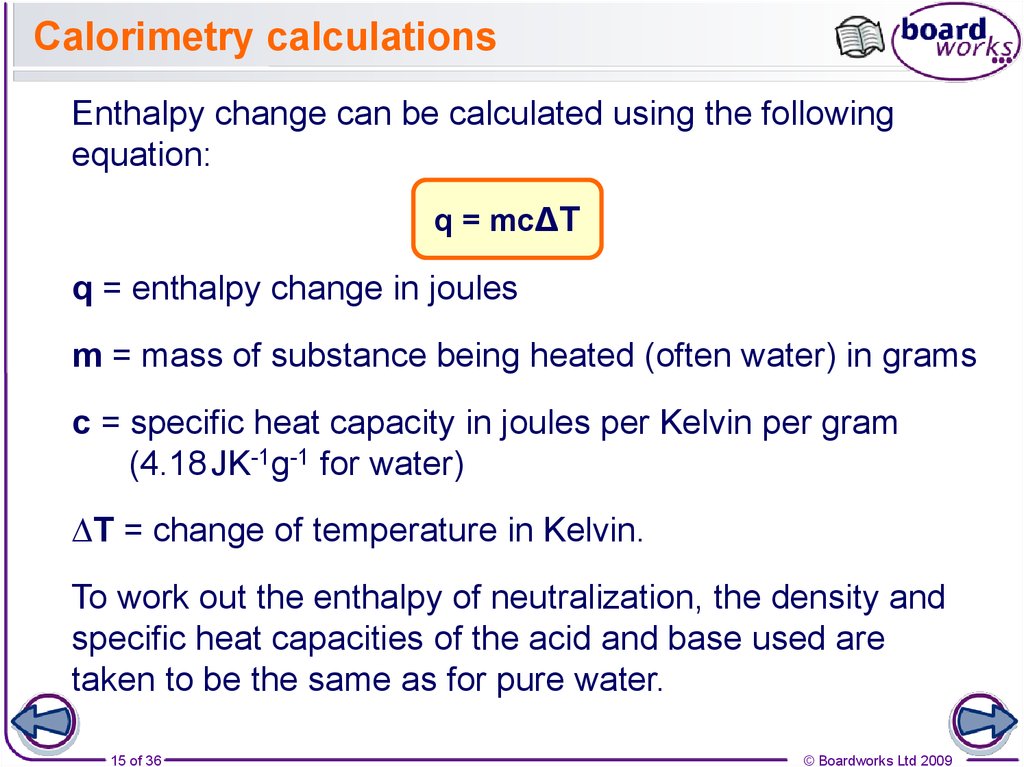
How To Calculate Q In Calorimetry . Then use equation \(\pageindex{2}\) to determine the heat capacity of the calorimeter. To use calorimetric data to calculate enthalpy changes. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. Equation for calculating energy transferred in a calorimeter In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. Additionally, it can find the. Then use equation \(ref{5.5.9}\) to. It can analyze the heat exchange between up to 3 objects. The energy transferred as heat can be calculated by: We now introduce two concepts useful in describing heat flow and temperature change. The calorimetry calculator can help you solve complex calorimetry problems. Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). The heat capacity (c) of a body of matter. While working these problems, review the sections on coffee cup and bomb calorimetry and the laws of thermochemistry. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb.
from worksheetmediadwaum.z14.web.core.windows.net
To use calorimetric data to calculate enthalpy changes. Then use equation \(ref{5.5.9}\) to. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. We now introduce two concepts useful in describing heat flow and temperature change. The heat capacity (c) of a body of matter. Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). The energy transferred as heat can be calculated by: Additionally, it can find the. The calorimetry calculator can help you solve complex calorimetry problems. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data.
How To Calculate Calorimetry Problems
How To Calculate Q In Calorimetry To use calorimetric data to calculate enthalpy changes. To use calorimetric data to calculate enthalpy changes. Equation for calculating energy transferred in a calorimeter While working these problems, review the sections on coffee cup and bomb calorimetry and the laws of thermochemistry. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Then use equation \(\pageindex{2}\) to determine the heat capacity of the calorimeter. We now introduce two concepts useful in describing heat flow and temperature change. Then use equation \(ref{5.5.9}\) to. The calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. The heat capacity (c) of a body of matter. Additionally, it can find the. Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). The energy transferred as heat can be calculated by: In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 How To Calculate Q In Calorimetry Then use equation \(\pageindex{2}\) to determine the heat capacity of the calorimeter. Additionally, it can find the. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. To use calorimetric data to calculate enthalpy changes. The calorimetry calculator can help you solve complex calorimetry problems. While working these problems, review the sections on coffee cup and. How To Calculate Q In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 How To Calculate Q In Calorimetry In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. Then use equation \(\pageindex{2}\) to determine the heat capacity of the calorimeter. The calorimetry calculator can help you solve complex calorimetry problems. Additionally, it can find the. To use calorimetric. How To Calculate Q In Calorimetry.
From worksheetlistsog.z21.web.core.windows.net
How To Calculate Calorimetry Problems How To Calculate Q In Calorimetry The heat capacity (c) of a body of matter. To use calorimetric data to calculate enthalpy changes. Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). The calorimetry calculator can help you solve complex calorimetry problems. The energy transferred as heat can be calculated by: Calculate the value of q rxn. How To Calculate Q In Calorimetry.
From www.wizeprep.com
Calorimetry Wize University Chemistry Textbook Wizeprep How To Calculate Q In Calorimetry Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Additionally, it can find the. Then use equation \(\pageindex{2}\) to determine the heat capacity of the calorimeter. The calorimetry calculator can help you solve complex calorimetry problems. The heat capacity (c) of a body of matter. While working these problems,. How To Calculate Q In Calorimetry.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe How To Calculate Q In Calorimetry The energy transferred as heat can be calculated by: Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). It can analyze the heat exchange between up to 3 objects. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. Calculate the value of q rxn for benzoic. How To Calculate Q In Calorimetry.
From studyminidiort.z21.web.core.windows.net
Heat And Calorimetry Worksheets How To Calculate Q In Calorimetry The calorimetry calculator can help you solve complex calorimetry problems. The heat capacity (c) of a body of matter. Additionally, it can find the. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating. How To Calculate Q In Calorimetry.
From www.youtube.com
Calorimetry Part 2 calculations YouTube How To Calculate Q In Calorimetry It can analyze the heat exchange between up to 3 objects. The calorimetry calculator can help you solve complex calorimetry problems. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. The heat capacity (c) of a body of matter.. How To Calculate Q In Calorimetry.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent How To Calculate Q In Calorimetry Then use equation \(ref{5.5.9}\) to. To use calorimetric data to calculate enthalpy changes. The energy transferred as heat can be calculated by: Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). Equation for calculating energy transferred in a calorimeter While working these problems, review the sections on coffee cup and bomb. How To Calculate Q In Calorimetry.
From www.youtube.com
Calorimetry Q=cmΔT ; Q=mL Numericals Class 10 ICSE Physics How To Calculate Q In Calorimetry Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). We now introduce two concepts useful in describing heat flow and temperature change. It can analyze the heat exchange between up to 3 objects. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. In a calorimetric determination,. How To Calculate Q In Calorimetry.
From worksheetlistsog.z21.web.core.windows.net
How To Calculate Calorimetry Problems How To Calculate Q In Calorimetry We now introduce two concepts useful in describing heat flow and temperature change. Additionally, it can find the. The energy transferred as heat can be calculated by: In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. These problems demonstrate. How To Calculate Q In Calorimetry.
From joiolgume.blob.core.windows.net
Calorimetry Heat Calculations at Brenda Salzer blog How To Calculate Q In Calorimetry Equation for calculating energy transferred in a calorimeter Additionally, it can find the. We now introduce two concepts useful in describing heat flow and temperature change. To use calorimetric data to calculate enthalpy changes. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its. How To Calculate Q In Calorimetry.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter How To Calculate Q In Calorimetry Additionally, it can find the. Equation for calculating energy transferred in a calorimeter Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). The heat capacity (c) of a body of matter. Then use equation \(\pageindex{2}\) to determine the heat capacity of the calorimeter. These problems demonstrate how to calculate heat transfer. How To Calculate Q In Calorimetry.
From joiddcrwa.blob.core.windows.net
What Is Calorimetry Example at Donovan blog How To Calculate Q In Calorimetry The energy transferred as heat can be calculated by: In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. Calculate the value of q rxn for. How To Calculate Q In Calorimetry.
From printablecampusreises.z21.web.core.windows.net
How To Calculate Calorimetry Problems How To Calculate Q In Calorimetry The energy transferred as heat can be calculated by: Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. To use calorimetric data to calculate enthalpy changes. Then use equation \(ref{5.5.9}\) to. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. Additionally, it can find. How To Calculate Q In Calorimetry.
From courses.lumenlearning.com
Calorimetry General Chemistry How To Calculate Q In Calorimetry We now introduce two concepts useful in describing heat flow and temperature change. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by. How To Calculate Q In Calorimetry.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube How To Calculate Q In Calorimetry The calorimetry calculator can help you solve complex calorimetry problems. To use calorimetric data to calculate enthalpy changes. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. Then use equation \(ref{5.5.9}\) to. We now introduce. How To Calculate Q In Calorimetry.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry How To Calculate Q In Calorimetry Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). While working these problems, review the sections on coffee cup and bomb calorimetry and the laws of thermochemistry. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. In a calorimetric determination, either (a) an exothermic process occurs. How To Calculate Q In Calorimetry.
From joiaygdte.blob.core.windows.net
How To Use A Calorimeter Step By Step at Albert Jones blog How To Calculate Q In Calorimetry Equation for calculating energy transferred in a calorimeter These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. Additionally, it can find the. Then use equation \(ref{5.5.9}\) to. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or. How To Calculate Q In Calorimetry.
From quizzmagicsydney.z13.web.core.windows.net
How To Calculate Calorimetry Problems How To Calculate Q In Calorimetry These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. To use calorimetric data to calculate enthalpy changes. It can analyze the heat exchange between up to 3 objects. While working these problems, review the sections on coffee cup and bomb calorimetry and the laws of thermochemistry. Calculate the value of \(q_{rxn}\) for benzoic acid by. How To Calculate Q In Calorimetry.
From studydbpetro.z4.web.core.windows.net
How To Calculate Calorimetry Problems How To Calculate Q In Calorimetry To use calorimetric data to calculate enthalpy changes. Additionally, it can find the. The heat capacity (c) of a body of matter. The calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its. How To Calculate Q In Calorimetry.
From printablezonemarrow.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry How To Calculate Q In Calorimetry Additionally, it can find the. To use calorimetric data to calculate enthalpy changes. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). The calorimetry calculator can help you solve complex calorimetry. How To Calculate Q In Calorimetry.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool How To Calculate Q In Calorimetry The calorimetry calculator can help you solve complex calorimetry problems. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. Additionally, it can find the. The energy transferred as heat can be calculated by: Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). It can analyze the. How To Calculate Q In Calorimetry.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry How To Calculate Q In Calorimetry It can analyze the heat exchange between up to 3 objects. The heat capacity (c) of a body of matter. The calorimetry calculator can help you solve complex calorimetry problems. While working these problems, review the sections on coffee cup and bomb calorimetry and the laws of thermochemistry. Additionally, it can find the. Then use equation \(ref{5.5.9}\) to. Calculate the. How To Calculate Q In Calorimetry.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog How To Calculate Q In Calorimetry It can analyze the heat exchange between up to 3 objects. Additionally, it can find the. Equation for calculating energy transferred in a calorimeter Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). The energy transferred as heat can be calculated by: To use calorimetric data to calculate enthalpy changes. The. How To Calculate Q In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 How To Calculate Q In Calorimetry Equation for calculating energy transferred in a calorimeter To use calorimetric data to calculate enthalpy changes. The energy transferred as heat can be calculated by: These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. The. How To Calculate Q In Calorimetry.
From users.highland.edu
Calorimetry How To Calculate Q In Calorimetry It can analyze the heat exchange between up to 3 objects. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. While working these problems, review the sections on coffee cup and bomb calorimetry and the laws of thermochemistry. Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its. How To Calculate Q In Calorimetry.
From joiolgume.blob.core.windows.net
Calorimetry Heat Calculations at Brenda Salzer blog How To Calculate Q In Calorimetry The heat capacity (c) of a body of matter. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). To use calorimetric. How To Calculate Q In Calorimetry.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube How To Calculate Q In Calorimetry These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. While working these problems, review the sections on coffee cup and bomb calorimetry and the laws of thermochemistry. The energy transferred as heat can be calculated by: Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). The. How To Calculate Q In Calorimetry.
From dxoazusse.blob.core.windows.net
Food Calorimetry Equation at Jesse Freeman blog How To Calculate Q In Calorimetry Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. While working these problems, review the sections on coffee cup and bomb. How To Calculate Q In Calorimetry.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube How To Calculate Q In Calorimetry Equation for calculating energy transferred in a calorimeter The calorimetry calculator can help you solve complex calorimetry problems. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. Additionally, it can find the. Calculate the value of q rxn for. How To Calculate Q In Calorimetry.
From joiwmxbys.blob.core.windows.net
Calorimetry With Two Solutions at Angelica Kirkland blog How To Calculate Q In Calorimetry Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Equation for calculating energy transferred in a calorimeter In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. The energy. How To Calculate Q In Calorimetry.
From classlibrarycordeiro.z21.web.core.windows.net
How To Calculate Calorimetry Problems How To Calculate Q In Calorimetry We now introduce two concepts useful in describing heat flow and temperature change. Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). The heat capacity (c) of a body of matter. It can analyze the heat exchange between up to 3 objects. In a calorimetric determination, either (a) an exothermic process. How To Calculate Q In Calorimetry.
From worksheetmediadwaum.z14.web.core.windows.net
How To Calculate Calorimetry Problems How To Calculate Q In Calorimetry These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data. Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). Equation for calculating energy transferred in a calorimeter In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. How To Calculate Q In Calorimetry.
From klaxzllai.blob.core.windows.net
Calorimetry Equation Examples at Grace Smallwood blog How To Calculate Q In Calorimetry Equation for calculating energy transferred in a calorimeter Then use equation \(ref{5.5.9}\) to. While working these problems, review the sections on coffee cup and bomb calorimetry and the laws of thermochemistry. We now introduce two concepts useful in describing heat flow and temperature change. The energy transferred as heat can be calculated by: Calculate the value of q rxn for. How To Calculate Q In Calorimetry.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry How To Calculate Q In Calorimetry Calculate the value of \(q_{rxn}\) for benzoic acid by multiplying the mass of benzoic acid by its \(δh_{comb}\). Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. To use calorimetric data to calculate enthalpy changes. These problems demonstrate how to calculate heat transfer and enthalpy change using calorimeter data.. How To Calculate Q In Calorimetry.