Dilution Examples In Real Life . The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. how to make a dilution. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. learn how to calculate and perform dilutions using different formulas and methods for biological science. the dilution formula can be used to create equations to figure out how to dilute a solution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. when a solution contains a relatively small amount of solute, it is said to be dilute; For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. If you do not know them. air, for example, is a solution. On the other hand, a solution with a relatively large amount of solute is said. Follow these five steps to make a dilution:
from labpedia.net
a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. the dilution formula can be used to create equations to figure out how to dilute a solution. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Follow these five steps to make a dilution: air, for example, is a solution. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. when a solution contains a relatively small amount of solute, it is said to be dilute; learn how to calculate and perform dilutions using different formulas and methods for biological science. If you do not know them. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a.
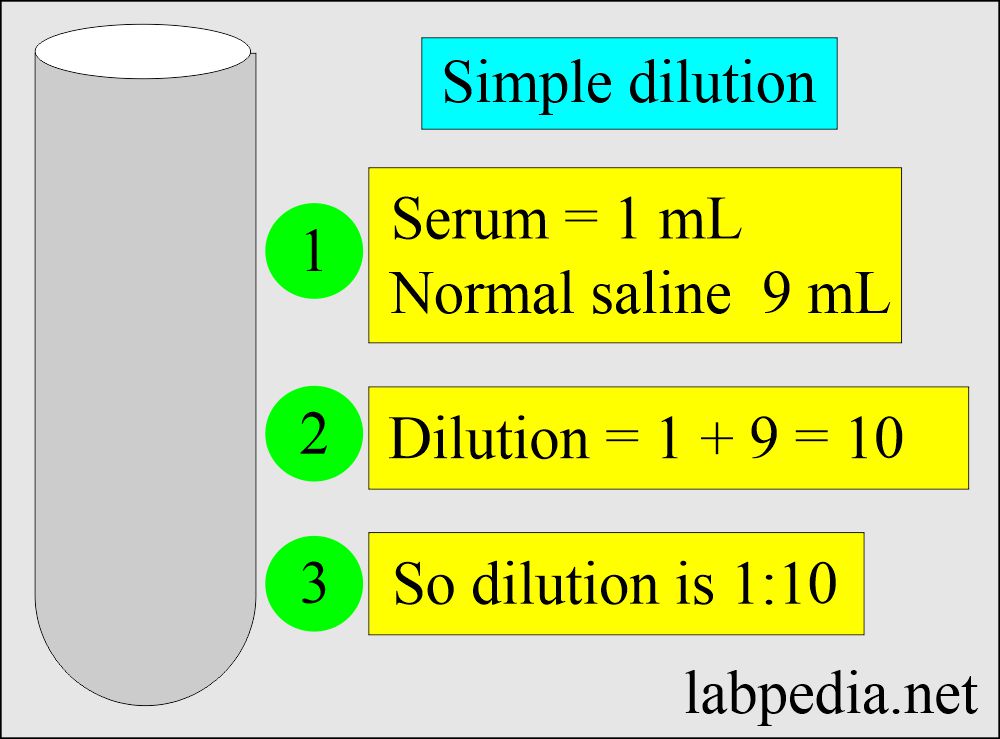
Solutions Part 1 Solutions Preparation used in Clinical Laboratory
Dilution Examples In Real Life For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. On the other hand, a solution with a relatively large amount of solute is said. The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. the dilution formula can be used to create equations to figure out how to dilute a solution. learn how to calculate and perform dilutions using different formulas and methods for biological science. If you do not know them. how to make a dilution. air, for example, is a solution. Follow these five steps to make a dilution: Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. when a solution contains a relatively small amount of solute, it is said to be dilute; For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Examples In Real Life a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. On the other hand, a solution with a relatively large amount of solute is said. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Follow these five steps to make a dilution:. Dilution Examples In Real Life.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Dilution Examples In Real Life Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. Follow these five steps to make a dilution: the dilution formula can be used to create equations. Dilution Examples In Real Life.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Examples In Real Life when a solution contains a relatively small amount of solute, it is said to be dilute; a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. On the other hand, a solution. Dilution Examples In Real Life.
From www.slideserve.com
PPT Dilution PowerPoint Presentation, free download ID6016027 Dilution Examples In Real Life The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. how to make a dilution. the dilution formula can be used to create equations to figure out how to dilute a solution. Follow these five steps to make a dilution: learn how to calculate and perform. Dilution Examples In Real Life.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Examples In Real Life the dilution formula can be used to create equations to figure out how to dilute a solution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Follow these five steps to make a dilution: If you do not know them. If you live near a lake, a river,. Dilution Examples In Real Life.
From www.slideserve.com
PPT Dilution and Spectroscopy Lab PowerPoint Presentation, free Dilution Examples In Real Life when a solution contains a relatively small amount of solute, it is said to be dilute; For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. On the other hand, a solution with a relatively large amount of solute is said. the dilution formula can be used to create equations. Dilution Examples In Real Life.
From hxegqvmtm.blob.core.windows.net
Dilution Method Definition at Ernest Alcazar blog Dilution Examples In Real Life air, for example, is a solution. when a solution contains a relatively small amount of solute, it is said to be dilute; On the other hand, a solution with a relatively large amount of solute is said. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o. Dilution Examples In Real Life.
From www.toppr.com
Dilution Formula Definition, Concepts and Examples Dilution Examples In Real Life the dilution formula can be used to create equations to figure out how to dilute a solution. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent.. Dilution Examples In Real Life.
From gioartpaa.blob.core.windows.net
What Are Dilutions Chemistry at Julian Smith blog Dilution Examples In Real Life On the other hand, a solution with a relatively large amount of solute is said. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. when a solution contains a relatively small amount of solute, it is said to be dilute; air, for example, is a solution. . Dilution Examples In Real Life.
From giokewoed.blob.core.windows.net
How Does Dilution Work Chemistry at Margaret Gamble blog Dilution Examples In Real Life Follow these five steps to make a dilution: If you do not know them. The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. the dilution formula can. Dilution Examples In Real Life.
From gioartpaa.blob.core.windows.net
What Are Dilutions Chemistry at Julian Smith blog Dilution Examples In Real Life learn how to calculate and perform dilutions using different formulas and methods for biological science. air, for example, is a solution. The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. If you do not know them. a dilute solution is one in which there is. Dilution Examples In Real Life.
From www.answersarena.com
[Solved] Dilution diagrams can be very helpful in organiz Dilution Examples In Real Life a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. If you do not know them. learn how to calculate and perform dilutions using different formulas and methods for biological science. how to make a dilution. when a solution contains a relatively small amount of solute, it. Dilution Examples In Real Life.
From www.youtube.com
Serial Dilution Technique For Microbiological & Chemical Analysis Dilution Examples In Real Life Follow these five steps to make a dilution: The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. If you do not know them. how to make a dilution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the. Dilution Examples In Real Life.
From facts.net
13 Surprising Facts About Dilution Dilution Examples In Real Life If you do not know them. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. On the other hand, a solution with a relatively large amount. Dilution Examples In Real Life.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Examples In Real Life air, for example, is a solution. the dilution formula can be used to create equations to figure out how to dilute a solution. If you do not know them. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. a dilute solution. Dilution Examples In Real Life.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Examples In Real Life how to make a dilution. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. If you live near a lake, a river, or an ocean, that body of water is not pure h. Dilution Examples In Real Life.
From fasrvs530.weebly.com
Techniques Of Serial Dilution fasrvs Dilution Examples In Real Life The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. when a solution contains a relatively small amount of solute, it is said to be dilute; how to make a dilution. learn how to calculate and perform dilutions using different formulas and methods for biological science.. Dilution Examples In Real Life.
From exytjhift.blob.core.windows.net
Dilute Solution Examples at Steven Wiseman blog Dilution Examples In Real Life Follow these five steps to make a dilution: when a solution contains a relatively small amount of solute, it is said to be dilute; If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. The water from the melting ice increases the volume of. Dilution Examples In Real Life.
From www.youtube.com
Dilution 101 Calculation And Examples YouTube Dilution Examples In Real Life The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. If you do not know them. the dilution formula can be used to create equations to figure out how to dilute a solution. If you live near a lake, a river, or an ocean, that body of water. Dilution Examples In Real Life.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution Examples In Real Life when a solution contains a relatively small amount of solute, it is said to be dilute; air, for example, is a solution. The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. On the other hand, a solution with a relatively large amount of solute is said.. Dilution Examples In Real Life.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilution Examples In Real Life If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. For example, we might say that a glass of iced tea becomes increasingly diluted. Dilution Examples In Real Life.
From www.slideserve.com
PPT Dilutions PowerPoint Presentation, free download ID226520 Dilution Examples In Real Life Follow these five steps to make a dilution: If you do not know them. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. learn how to calculate and perform dilutions using different formulas and methods for biological science. the dilution formula can be used to create equations. Dilution Examples In Real Life.
From www.youtube.com
Serial Dilution Required Practical Revision for Biology and Chemistry Dilution Examples In Real Life The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. the dilution formula can be used to create equations to figure out how. Dilution Examples In Real Life.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer Dilution Examples In Real Life If you do not know them. when a solution contains a relatively small amount of solute, it is said to be dilute; Follow these five steps to make a dilution: If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. air, for example,. Dilution Examples In Real Life.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Examples In Real Life Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. when a solution contains a relatively small amount of solute, it is said to be dilute; how to make a dilution. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. learn. Dilution Examples In Real Life.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Examples In Real Life when a solution contains a relatively small amount of solute, it is said to be dilute; If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. a dilute solution is one in which there is a relatively small amount of solute dissolved in. Dilution Examples In Real Life.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube Dilution Examples In Real Life On the other hand, a solution with a relatively large amount of solute is said. If you do not know them. the dilution formula can be used to create equations to figure out how to dilute a solution. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o. Dilution Examples In Real Life.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Examples In Real Life the dilution formula can be used to create equations to figure out how to dilute a solution. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Follow these five steps to make a. Dilution Examples In Real Life.
From www.youtube.com
Video Demonstration Dilution Part 2 YouTube Dilution Examples In Real Life when a solution contains a relatively small amount of solute, it is said to be dilute; The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but. Dilution Examples In Real Life.
From www.youtube.com
Unit 8.7 Dilution and Examples YouTube Dilution Examples In Real Life air, for example, is a solution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. how to make a dilution. Follow these five steps to make a dilution: If you live near a lake, a river, or an ocean, that body of water is not pure h. Dilution Examples In Real Life.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution Examples In Real Life On the other hand, a solution with a relatively large amount of solute is said. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. when. Dilution Examples In Real Life.
From adropofthis.blogspot.com
A Drop of This The Solution for Dilution Dilution Examples In Real Life For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. If you do not know them. when a solution contains a relatively small amount of solute, it is said to be dilute; how to make a dilution. learn how to calculate and perform dilutions using different formulas and methods. Dilution Examples In Real Life.
From www.researchgate.net
The 12 dilution procedure produces plenty of solution to measure but Dilution Examples In Real Life The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced. learn how to calculate and perform dilutions using different formulas and methods for biological science. how to make a dilution. when a solution contains a relatively small amount of solute, it is said to be dilute;. Dilution Examples In Real Life.
From fyoamjyfe.blob.core.windows.net
What Is Dilution In Chemistry Class 10 at Irene Ochoa blog Dilution Examples In Real Life If you do not know them. If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. air, for example, is a solution. when a solution contains a relatively small amount of solute, it is said to be dilute; a dilute solution is. Dilution Examples In Real Life.
From www.expressmicroscience.co.uk
Why are dilutions important? Express Micro Science Dilution Examples In Real Life If you live near a lake, a river, or an ocean, that body of water is not pure h 2 o but most probably a. the dilution formula can be used to create equations to figure out how to dilute a solution. a dilute solution is one in which there is a relatively small amount of solute dissolved. Dilution Examples In Real Life.