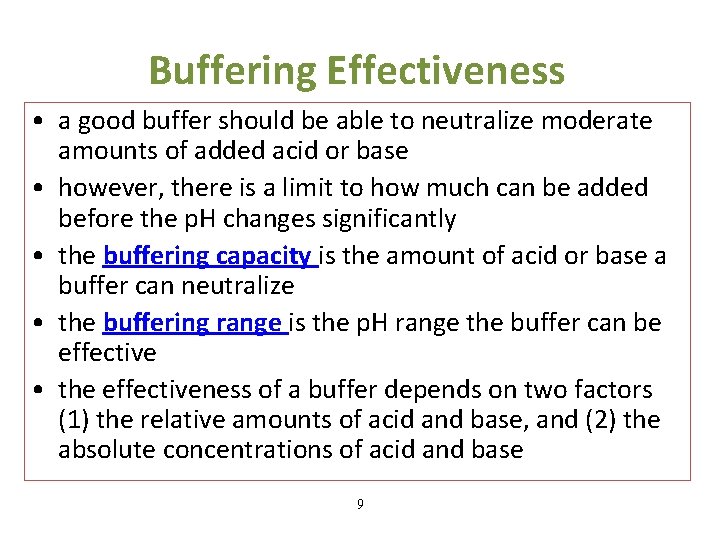
What Makes A Good Buffer In Chemistry . a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. Adding a strong electrolyte that. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize. a good buffer mixture should have about equal concentrations of both of its components. buffers are solutions that resist a change in ph after adding an acid or a base.
from slidetodoc.com
Adding a strong electrolyte that. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a good buffer mixture should have about equal concentrations of both of its components.
Introduction to Buffers These solutions contain relatively high
What Makes A Good Buffer In Chemistry Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). buffers are solutions that resist a change in ph after adding an acid or a base. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize. a good buffer mixture should have about equal concentrations of both of its components. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Adding a strong electrolyte that.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts What Makes A Good Buffer In Chemistry buffers are solutions that resist a change in ph after adding an acid or a base. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize. Adding a strong electrolyte that. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). . What Makes A Good Buffer In Chemistry.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Makes A Good Buffer In Chemistry a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. It is able to neutralize. buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer mixture should have about equal concentrations of both of its. What Makes A Good Buffer In Chemistry.
From www.chemicals.co.uk
What Makes A Good Buffer In Chemistry? The Chemistry Blog What Makes A Good Buffer In Chemistry buffers are solutions that resist a change in ph after adding an acid or a base. Adding a strong electrolyte that. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a solution that maintains the stability of a system’s ph level when adding small. What Makes A Good Buffer In Chemistry.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses What Makes A Good Buffer In Chemistry a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. Adding a strong electrolyte that. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. buffers are solutions that resist a change. What Makes A Good Buffer In Chemistry.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Makes A Good Buffer In Chemistry a good buffer mixture should have about equal concentrations of both of its components. a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. buffers are solutions that resist a change in ph after adding an acid or a base. Adding a strong electrolyte. What Makes A Good Buffer In Chemistry.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 What Makes A Good Buffer In Chemistry a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a good buffer solution in chemistry is a solution that maintains the ph level of a. What Makes A Good Buffer In Chemistry.
From mydigitalkemistry.com
What is a buffer solution simple definition? Free Chemistry Learning What Makes A Good Buffer In Chemistry a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Adding a strong electrolyte that. a good buffer mixture should have about equal concentrations of both of its components. It is able to neutralize. a good buffer solution in chemistry is a solution that maintains. What Makes A Good Buffer In Chemistry.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid What Makes A Good Buffer In Chemistry It is able to neutralize. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a good buffer mixture should have about equal concentrations of both. What Makes A Good Buffer In Chemistry.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Makes A Good Buffer In Chemistry a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Adding a strong electrolyte that. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). It is able to neutralize. buffers are solutions that resist a change in ph after adding an acid or a base. . What Makes A Good Buffer In Chemistry.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Makes A Good Buffer In Chemistry Adding a strong electrolyte that. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. buffers are solutions that resist a change. What Makes A Good Buffer In Chemistry.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Makes A Good Buffer In Chemistry Adding a strong electrolyte that. a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. a good buffer mixture should have about equal concentrations of both of its components. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). a buffer is. What Makes A Good Buffer In Chemistry.
From fyohrbtxu.blob.core.windows.net
What Is A Good Buffer System at James Norris blog What Makes A Good Buffer In Chemistry It is able to neutralize. a good buffer mixture should have about equal concentrations of both of its components. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. buffers are solutions. What Makes A Good Buffer In Chemistry.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions What Makes A Good Buffer In Chemistry Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer mixture should have about equal concentrations of both of its components. Adding a strong electrolyte that. It is able to neutralize. a buffer is a solution. What Makes A Good Buffer In Chemistry.
From www.slideshare.net
Buffer system What Makes A Good Buffer In Chemistry a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer mixture should have about equal concentrations of both of its components. It is able to neutralize. Buffers contain a. What Makes A Good Buffer In Chemistry.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions What Makes A Good Buffer In Chemistry It is able to neutralize. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer mixture should have about equal concentrations of both of its components.. What Makes A Good Buffer In Chemistry.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Makes A Good Buffer In Chemistry It is able to neutralize. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Adding a strong electrolyte that. a buffer is a solution that maintains the stability of a system’s ph level when adding small. What Makes A Good Buffer In Chemistry.
From www.slideserve.com
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242 What Makes A Good Buffer In Chemistry buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer mixture should have about equal concentrations of both of its components. a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. Adding a strong electrolyte. What Makes A Good Buffer In Chemistry.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide What Makes A Good Buffer In Chemistry a good buffer mixture should have about equal concentrations of both of its components. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). buffers are solutions that resist a change in ph after adding an acid or a base. It is able to neutralize. a buffer is a solution that maintains the stability of. What Makes A Good Buffer In Chemistry.
From www.pinterest.com
Buffer Study chemistry, Chemistry classroom, Organic chemistry study What Makes A Good Buffer In Chemistry It is able to neutralize. a good buffer mixture should have about equal concentrations of both of its components. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer solution in chemistry is a solution that. What Makes A Good Buffer In Chemistry.
From www.chemicals.co.uk
What Makes A Good Buffer In Chemistry? The Chemistry Blog What Makes A Good Buffer In Chemistry buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. a good buffer mixture should have about equal concentrations of both of its components. Adding a strong electrolyte. What Makes A Good Buffer In Chemistry.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Makes A Good Buffer In Chemistry buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. a buffer is a solution that can resist ph change upon the addition of an acidic or basic. What Makes A Good Buffer In Chemistry.
From chemistryguru.com.sg
What is a Buffer Solution? What Makes A Good Buffer In Chemistry buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities. What Makes A Good Buffer In Chemistry.
From www.ck12.org
Buffer Solutions Example 1 ( Video ) Chemistry CK12 Foundation What Makes A Good Buffer In Chemistry a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. It is able to neutralize. a buffer is a solution that maintains the stability of. What Makes A Good Buffer In Chemistry.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 What Makes A Good Buffer In Chemistry It is able to neutralize. Adding a strong electrolyte that. buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer mixture should have about equal concentrations of both of its components. a buffer is a solution that maintains the stability of a system’s ph level when adding small. What Makes A Good Buffer In Chemistry.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Makes A Good Buffer In Chemistry a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a good buffer mixture should have about equal concentrations of both of. What Makes A Good Buffer In Chemistry.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation What Makes A Good Buffer In Chemistry Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Adding a strong electrolyte. What Makes A Good Buffer In Chemistry.
From albertachemicals.com
What Makes a Good Buffer in Chemistry Essential Characteristics What Makes A Good Buffer In Chemistry It is able to neutralize. Adding a strong electrolyte that. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. buffers are solutions that resist a change in ph after adding an acid or a base. a buffer is a solution that can resist ph. What Makes A Good Buffer In Chemistry.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Makes A Good Buffer In Chemistry buffers are solutions that resist a change in ph after adding an acid or a base. Adding a strong electrolyte that. It is able to neutralize. a good buffer mixture should have about equal concentrations of both of its components. a good buffer solution in chemistry is a solution that maintains the ph level of a system. What Makes A Good Buffer In Chemistry.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Makes A Good Buffer In Chemistry Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). It is able to neutralize. Adding a strong electrolyte that. buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer mixture should have about equal concentrations of both of its components. a buffer is a solution. What Makes A Good Buffer In Chemistry.
From www.chemicals.co.uk
What Is A Buffer Solution? The Chemistry Blog What Makes A Good Buffer In Chemistry buffers are solutions that resist a change in ph after adding an acid or a base. a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities. What Makes A Good Buffer In Chemistry.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Makes A Good Buffer In Chemistry a good buffer solution in chemistry is a solution that maintains the ph level of a system and resists changes in ion concentration. Adding a strong electrolyte that. It is able to neutralize. a good buffer mixture should have about equal concentrations of both of its components. Buffers contain a weak acid (\(ha\)) and its conjugate weak base. What Makes A Good Buffer In Chemistry.
From 2012books.lardbucket.org
Buffers What Makes A Good Buffer In Chemistry a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a good buffer mixture should have about equal concentrations of both of its components. Adding a strong electrolyte that. a good buffer solution in chemistry is a solution that maintains the ph level of a. What Makes A Good Buffer In Chemistry.
From www.sliderbase.com
Buffers Presentation Chemistry What Makes A Good Buffer In Chemistry a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. buffers are solutions that resist a change in ph after adding an acid or a base.. What Makes A Good Buffer In Chemistry.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Makes A Good Buffer In Chemistry Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). It is able to neutralize. Adding a strong electrolyte that. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a good buffer solution in chemistry is a solution that maintains the ph level. What Makes A Good Buffer In Chemistry.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation What Makes A Good Buffer In Chemistry a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Adding a strong electrolyte that. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)).. What Makes A Good Buffer In Chemistry.