Function Of Buffer Solution In Experiment . A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Biochemical experiments routinely require a buffer. It is able to neutralize small. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph. What is a buffer solution?
from chem.libretexts.org
A buffer solution is one which resists changes in ph. This page describes simple acidic and alkaline buffer solutions and explains how they work. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Biochemical experiments routinely require a buffer. It is able to neutralize small. What is a buffer solution? A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or.
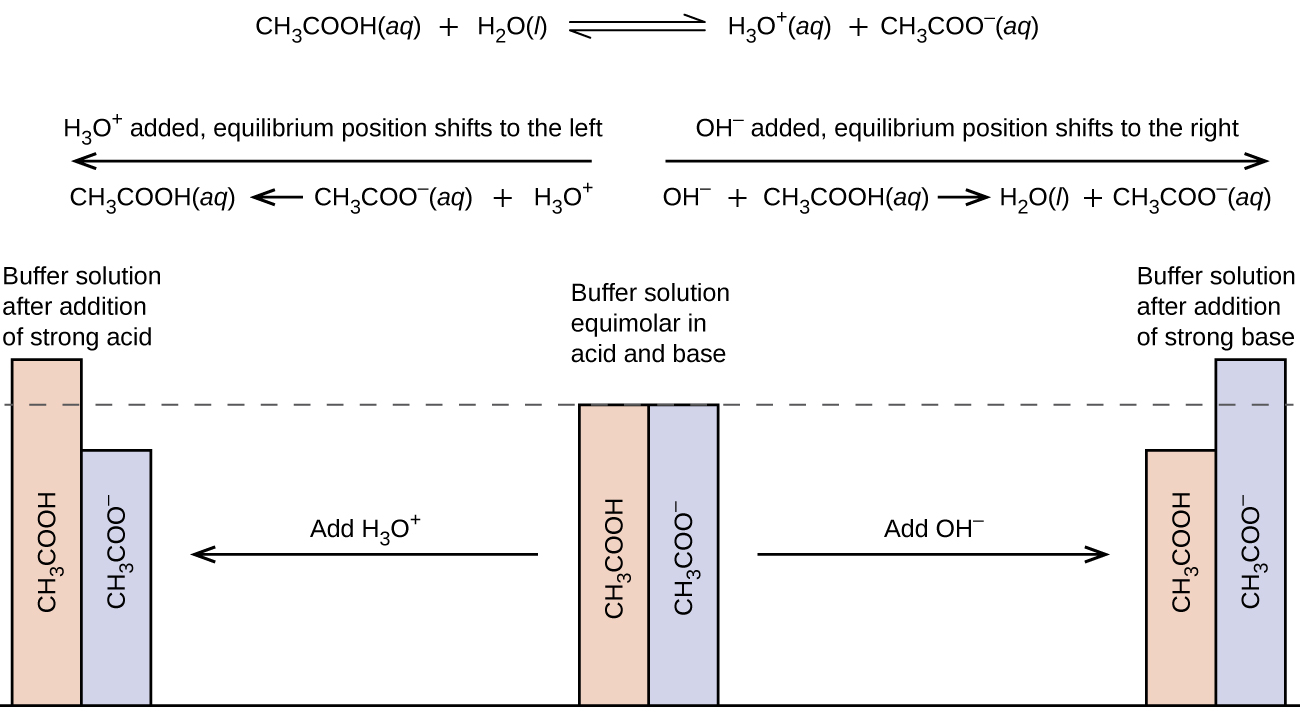
14.6 Buffers Chemistry LibreTexts
Function Of Buffer Solution In Experiment This page describes simple acidic and alkaline buffer solutions and explains how they work. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer solution is one which resists changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This page describes simple acidic and alkaline buffer solutions and explains how they work. Biochemical experiments routinely require a buffer. What is a buffer solution? A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small.
From www.ck12.org
Buffer Solutions Example 1 ( Video ) Chemistry CK12 Foundation Function Of Buffer Solution In Experiment It is able to neutralize small. In this laboratory you will cover the basics of buffer preparation and test the buffering. Biochemical experiments routinely require a buffer. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that. Function Of Buffer Solution In Experiment.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Function Of Buffer Solution In Experiment A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Biochemical experiments routinely require a buffer. It is able to neutralize small. In this laboratory you will cover the basics of buffer preparation and test the buffering. A mixture of a weak acid and its conjugate base (or a mixture of. Function Of Buffer Solution In Experiment.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 Function Of Buffer Solution In Experiment This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution is one which resists changes in ph. Biochemical experiments routinely require a buffer. It is able to neutralize small. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer is a solution that can resist ph. Function Of Buffer Solution In Experiment.
From www.youtube.com
TRU Chemistry Labs Experiment Titration Curves Part B Buffer Systems Function Of Buffer Solution In Experiment Biochemical experiments routinely require a buffer. What is a buffer solution? A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. Function Of Buffer Solution In Experiment.
From www.mheducation.com
What is McGraw Hill Virtual Labs? McGraw Hill Higher Education Function Of Buffer Solution In Experiment A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Biochemical experiments routinely require a buffer. This page describes simple acidic and alkaline buffer solutions and explains how they. Function Of Buffer Solution In Experiment.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Function Of Buffer Solution In Experiment In this laboratory you will cover the basics of buffer preparation and test the buffering. Biochemical experiments routinely require a buffer. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. This page describes simple acidic and alkaline buffer solutions and explains how. Function Of Buffer Solution In Experiment.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID1031156 Function Of Buffer Solution In Experiment A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph. Biochemical experiments routinely require a buffer. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is a. Function Of Buffer Solution In Experiment.
From www.thinkswap.com
Buffer Solution MF008 General Chemistry I UCSI Thinkswap Function Of Buffer Solution In Experiment A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In this laboratory you will cover the basics of buffer preparation and test the buffering. What is a buffer solution? This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a. Function Of Buffer Solution In Experiment.
From www.feedback-shop.co.uk
Buffer solutions Digital Protolysis equilibrium Acids and bases Function Of Buffer Solution In Experiment Biochemical experiments routinely require a buffer. A buffer solution is one which resists changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a solution that maintains the stability of a. Function Of Buffer Solution In Experiment.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses Function Of Buffer Solution In Experiment A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It is able to neutralize small. What is a buffer solution? A buffer solution is one which resists changes in ph. In this laboratory you will cover the basics of buffer preparation and. Function Of Buffer Solution In Experiment.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Function Of Buffer Solution In Experiment What is a buffer solution? This page describes simple acidic and alkaline buffer solutions and explains how they work. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding. Function Of Buffer Solution In Experiment.
From studylib.net
Buffer Solutions Function Of Buffer Solution In Experiment It is able to neutralize small. This page describes simple acidic and alkaline buffer solutions and explains how they work. In this laboratory you will cover the basics of buffer preparation and test the buffering. What is a buffer solution? Biochemical experiments routinely require a buffer. A buffer is a solution that can resist ph change upon the addition of. Function Of Buffer Solution In Experiment.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid Function Of Buffer Solution In Experiment This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Biochemical experiments routinely require a buffer. It is able to neutralize small. What is a buffer solution? A buffer solution is one which resists. Function Of Buffer Solution In Experiment.
From www.researchgate.net
Distribution of species as a function of pH of buffer solution for IVZ Function Of Buffer Solution In Experiment A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer solution is one which resists changes in ph. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In. Function Of Buffer Solution In Experiment.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 Function Of Buffer Solution In Experiment This page describes simple acidic and alkaline buffer solutions and explains how they work. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition. Function Of Buffer Solution In Experiment.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Function Of Buffer Solution In Experiment A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is a buffer solution? Biochemical experiments routinely require a buffer. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer solution is one which resists changes in ph. It is. Function Of Buffer Solution In Experiment.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Function Of Buffer Solution In Experiment A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Biochemical experiments routinely require a buffer. A mixture of a weak acid and its conjugate base (or a mixture. Function Of Buffer Solution In Experiment.
From www.tes.com
Buffer Solutions 3 (A Level Chemistry) Teaching Resources Function Of Buffer Solution In Experiment A buffer solution is one which resists changes in ph. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer solution? A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids. Function Of Buffer Solution In Experiment.
From studylib.net
experiment 16 buffer solutions Function Of Buffer Solution In Experiment A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a solution that maintains the stability of a system’s ph level when adding small. Function Of Buffer Solution In Experiment.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Function Of Buffer Solution In Experiment A buffer solution is one which resists changes in ph. This page describes simple acidic and alkaline buffer solutions and explains how they work. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that maintains the stability. Function Of Buffer Solution In Experiment.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Function Of Buffer Solution In Experiment A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer solution is one which resists changes in ph. It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level when adding small. Function Of Buffer Solution In Experiment.
From www.feedback-shop.co.uk
Buffer solutions Protolysis equilibrium Acids and bases Function Of Buffer Solution In Experiment This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer solution? A buffer solution is one which resists changes in ph. Biochemical experiments routinely require a buffer. A mixture of a weak acid. Function Of Buffer Solution In Experiment.
From www.youtube.com
EXPERIMENT Preparation of buffer solution and measurement of pH Function Of Buffer Solution In Experiment A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? A buffer solution is one which resists changes in ph. It is able to neutralize small. In this laboratory you will cover. Function Of Buffer Solution In Experiment.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Function Of Buffer Solution In Experiment What is a buffer solution? A buffer solution is one which resists changes in ph. Biochemical experiments routinely require a buffer. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer. Function Of Buffer Solution In Experiment.
From pdfprof.com
determining buffer capacity experiment Function Of Buffer Solution In Experiment A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Biochemical experiments routinely require a buffer. A buffer solution is one which resists changes in ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is. Function Of Buffer Solution In Experiment.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Function Of Buffer Solution In Experiment What is a buffer solution? A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. In this laboratory you will cover the basics of buffer preparation and test the buffering. Biochemical experiments routinely require a buffer. A buffer is a solution that can. Function Of Buffer Solution In Experiment.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Function Of Buffer Solution In Experiment A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In this laboratory you will cover the basics of buffer preparation and test the buffering. Biochemical experiments routinely require a buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or. Function Of Buffer Solution In Experiment.
From slidetodoc.com
EXPERIMENT 5 Preparation and Properties of Buffer Solution Function Of Buffer Solution In Experiment It is able to neutralize small. In this laboratory you will cover the basics of buffer preparation and test the buffering. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer solution? A. Function Of Buffer Solution In Experiment.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Function Of Buffer Solution In Experiment A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. This page describes simple acidic and alkaline buffer solutions and explains how they work. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer is a. Function Of Buffer Solution In Experiment.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy Function Of Buffer Solution In Experiment A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is one which resists changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer solution? In this laboratory you. Function Of Buffer Solution In Experiment.
From pdfprof.com
buffer capacity experiment procedure Function Of Buffer Solution In Experiment A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Biochemical experiments routinely require a buffer. This page describes simple acidic. Function Of Buffer Solution In Experiment.
From www.chegg.com
Solved Experiment 3 Preparation of Buffers Buffers play Function Of Buffer Solution In Experiment It is able to neutralize small. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is a buffer solution? A buffer solution is one which resists changes in ph. A buffer. Function Of Buffer Solution In Experiment.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Function Of Buffer Solution In Experiment In this laboratory you will cover the basics of buffer preparation and test the buffering. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Biochemical experiments routinely require a buffer. What is a buffer solution? A buffer solution is one which resists changes in. Function Of Buffer Solution In Experiment.
From www.slideshare.net
2012 topic 18 2 buffer solutions Function Of Buffer Solution In Experiment It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer solution? Biochemical experiments routinely require a buffer. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer solution is one which resists changes in. Function Of Buffer Solution In Experiment.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Function Of Buffer Solution In Experiment In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. What is a buffer solution? A buffer is a solution that maintains the stability of a system’s ph level when. Function Of Buffer Solution In Experiment.