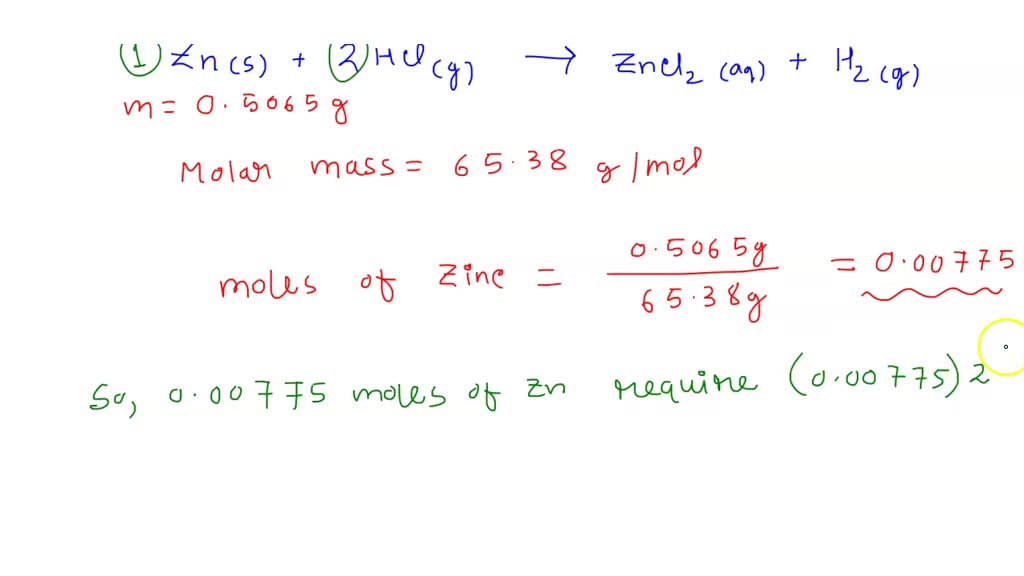Zinc Chloride Volume . For example, water is h. steps to calculate molar mass. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. The structure of a zncl 2 molecule is illustrated below. Zinc chloride is an inorganic binary salt. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. Write down the chemical formula of the compound. zinc chloride structure. zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. [ weight to volume | volume to. Cas registry number (cas rn):
from www.numerade.com
zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. zinc chloride structure. steps to calculate molar mass. For example, water is h. Cas registry number (cas rn): [ weight to volume | volume to. The structure of a zncl 2 molecule is illustrated below. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. Write down the chemical formula of the compound. Zinc chloride is an inorganic binary salt.
SOLVED In the laboratory YOu dissolve 22.6 g of zinc chloride in a
Zinc Chloride Volume The structure of a zncl 2 molecule is illustrated below. zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Cas registry number (cas rn): Write down the chemical formula of the compound. steps to calculate molar mass. The structure of a zncl 2 molecule is illustrated below. For example, water is h. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. [ weight to volume | volume to. zinc chloride structure. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. Zinc chloride is an inorganic binary salt.
From www.pw.live
Zinc Chloride Formula Zinc Chloride Volume zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. For example, water is h. zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. The structure of a zncl 2 molecule is illustrated below. Cas. Zinc Chloride Volume.
From paramountchemicals.com.au
Zinc Chloride Paramount Chemicals Zinc Chloride Volume The structure of a zncl 2 molecule is illustrated below. For example, water is h. Cas registry number (cas rn): zinc chloride structure. Write down the chemical formula of the compound. [ weight to volume | volume to. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent. Zinc Chloride Volume.
From camachem.com
What is Zinc Chloride and How to Buy Zinc Chloride? Zinc Chloride Volume [ weight to volume | volume to. steps to calculate molar mass. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. Zinc chloride is an inorganic binary salt. Write down the chemical formula of the compound. The structure of a zncl. Zinc Chloride Volume.
From dalkemcorp.com
Zinc Chloride Technical Grade 500 grams (G.W.) Store Online Zinc Chloride Volume zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. Cas registry number (cas rn): For example, water is h. The structure of a zncl 2 molecule is illustrated below. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve. Zinc Chloride Volume.
From www.indiamart.com
Zinc Chloride Solution Manufacturer from Sangrur Zinc Chloride Volume zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Zinc chloride is an inorganic binary salt. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. For example, water is h.. Zinc Chloride Volume.
From www.somersetsolders.com
AIM Solders Zinc Chloride Flux 500ml Zinc Chloride Volume zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. For example, water is h. steps to calculate molar mass. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. . Zinc Chloride Volume.
From www.flinnsci.com
Flinn Chemicals, Zinc Chloride Zinc Chloride Volume zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. steps to calculate molar mass. Write down the chemical formula of the compound. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in. Zinc Chloride Volume.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc Chloride Volume steps to calculate molar mass. Zinc chloride is an inorganic binary salt. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity). Zinc Chloride Volume.
From www.bonnymans.co.uk
Zinc Chloride ZINCC5 Powders Bonnymans Zinc Chloride Volume steps to calculate molar mass. Cas registry number (cas rn): The structure of a zncl 2 molecule is illustrated below. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. For example, water is h. Write down the chemical formula of the compound. zinc chloride. Zinc Chloride Volume.
From www.isolab.de
ISOLAB ZINC CHLORIDE CAS No 7646857 ISOLAB GmbH Zinc Chloride Volume [ weight to volume | volume to. The structure of a zncl 2 molecule is illustrated below. Write down the chemical formula of the compound. Cas registry number (cas rn): steps to calculate molar mass. zinc chloride structure. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of. Zinc Chloride Volume.
From www.shutterstock.com
Zinc Chloride Formula Zncl2 Cl2zn White ilustración de stock 769733368 Zinc Chloride Volume The structure of a zncl 2 molecule is illustrated below. [ weight to volume | volume to. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. Cas registry number (cas rn): to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have. Zinc Chloride Volume.
From first-labs.com
Zinc chloride Zinc Chloride Volume zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. zinc chloride structure. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. [ weight to volume | volume to. For example, water is h.. Zinc Chloride Volume.
From express.adobe.com
Zinc and Copper Chloride Zinc Chloride Volume Write down the chemical formula of the compound. For example, water is h. steps to calculate molar mass. [ weight to volume | volume to. zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. zinc chloride is a solution of ions indicated for use in total. Zinc Chloride Volume.
From www.toppr.com
How will you obtain zinc chloride from zinc sulphate? Zinc Chloride Volume The structure of a zncl 2 molecule is illustrated below. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or.. Zinc Chloride Volume.
From www.laboratoriumdiscounter.nl
Buy Zinc Chloride CAS 7646857 ? Pure zinc chloride CAS 7646857 in Zinc Chloride Volume to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. Zinc chloride is an inorganic binary salt. For example, water is h. zinc chloride structure. [ weight to volume | volume to. Write down the chemical formula of the compound. zinc. Zinc Chloride Volume.
From www.sciencecompany.com
Tech Grade Anhydrous Zinc Chloride, 500g for sale. Buy from The Science Zinc Chloride Volume steps to calculate molar mass. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. The structure of a zncl 2 molecule is illustrated below. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2. Zinc Chloride Volume.
From www.grainger.com
7646857, 136.29, Zinc Chloride, Granular, Reagent, ACS 6NPF3Z1055 Zinc Chloride Volume For example, water is h. The structure of a zncl 2 molecule is illustrated below. Zinc chloride is an inorganic binary salt. zinc chloride structure. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. to prepare 1000 ml of a 0.1 mol/l solution of. Zinc Chloride Volume.
From paramountchemicals.com.au
Zinc Chloride Paramount Chemicals Zinc Chloride Volume zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. zinc chloride structure. steps to calculate molar mass. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or.. Zinc Chloride Volume.
From commons.wikimedia.org
FileZincchloridextal3DSF.png Wikimedia Commons Zinc Chloride Volume Write down the chemical formula of the compound. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. steps to calculate molar mass. zinc chloride structure. zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid. Zinc Chloride Volume.
From www.researchgate.net
What is the proper way to prepare a zinc chloride containing buffer for Zinc Chloride Volume zinc chloride structure. Write down the chemical formula of the compound. Cas registry number (cas rn): [ weight to volume | volume to. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. Zinc chloride is an inorganic binary salt. steps to calculate molar mass.. Zinc Chloride Volume.
From www.somersetsolders.com
AIM Solders Zinc Chloride Flux 1 Litre Zinc Chloride Volume steps to calculate molar mass. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. [ weight to volume. Zinc Chloride Volume.
From www.sunshinetrading.co
Zinc Chloride Sunshine Trading Company Zinc Chloride Volume to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. Cas registry number (cas rn): zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Write down the chemical formula of the. Zinc Chloride Volume.
From www.npchem.co.th
ZINC CHLORIDE anhydrous, Lab, 500g, KemAus Zinc Chloride Volume zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. zinc chloride structure. The structure of a zncl 2. Zinc Chloride Volume.
From www.laballey.com
Zinc Chloride, 1M Lab Alley Zinc Chloride Volume to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Zinc chloride is an inorganic binary salt. zinc chloride is a. Zinc Chloride Volume.
From www.numerade.com
SOLVED In the laboratory YOu dissolve 22.6 g of zinc chloride in a Zinc Chloride Volume [ weight to volume | volume to. steps to calculate molar mass. zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Write down the chemical formula of the compound. The structure of a zncl 2 molecule is illustrated below. For example, water is h. Cas registry number. Zinc Chloride Volume.
From chemcraft.su
Zinc chloride, 98 pure chemcraft.su Zinc Chloride Volume zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. For example, water is h. Write down the chemical formula of the compound. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. [ weight to. Zinc Chloride Volume.
From www.indiamart.com
250 g Zinc Chloride Dry at best price in Pinjore ID 23826350562 Zinc Chloride Volume [ weight to volume | volume to. For example, water is h. zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. Cas registry number (cas rn): to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of. Zinc Chloride Volume.
From www.mcguffmedical.com
Zinc Trace Element, Chloride, 1mg/mL, SDV, 10mL Vial McGuff Medical Zinc Chloride Volume zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. Zinc chloride is an inorganic binary salt. zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. to prepare 1000 ml of a 0.1 mol/l. Zinc Chloride Volume.
From www.carolina.com
Zinc Chloride, Reagent Grade, 500 g Carolina Biological Supply Zinc Chloride Volume The structure of a zncl 2 molecule is illustrated below. Cas registry number (cas rn): [ weight to volume | volume to. Zinc chloride is an inorganic binary salt. For example, water is h. zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. zinc chloride is a. Zinc Chloride Volume.
From www.sigmaaldrich.co.th
ZINC CHLORIDE, 98+ Merck Life Sciences Thailand Zinc Chloride Volume to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. Zinc chloride is an inorganic binary salt. The structure of a zncl 2 molecule is illustrated below. zinc chloride structure. zinc chloride, represented by the chemical formula zncl 2, is a. Zinc Chloride Volume.
From www.youtube.com
How to Write the Formula for Zinc chloride (ZnCl2) YouTube Zinc Chloride Volume Zinc chloride is an inorganic binary salt. For example, water is h. Cas registry number (cas rn): The structure of a zncl 2 molecule is illustrated below. [ weight to volume | volume to. Write down the chemical formula of the compound. zinc chloride structure. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we. Zinc Chloride Volume.
From www.laballey.com
Buy Zinc Chloride, 1M Solution 35+ Bulk Sizes Zinc Chloride Volume to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. zinc chloride structure. Cas registry number (cas rn): The structure of a zncl 2 molecule is illustrated below. [ weight to volume | volume to. Zinc chloride is an inorganic binary salt.. Zinc Chloride Volume.
From www.chegg.com
Solved REPORT SHEET Chemical Formulas A, Zinc Chloride 1. Zinc Chloride Volume to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. Zinc chloride is an inorganic binary salt. The structure of a zncl 2 molecule is illustrated below. Cas registry number (cas rn): For example, water is h. Write down the chemical formula of. Zinc Chloride Volume.
From www.carolina.com
Zinc Chloride, 1.0 M Aqueous, Laboratory Grade, 500 mL Carolina Zinc Chloride Volume zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes. zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Write down the chemical formula of the compound. to prepare 1000 ml of a 0.1. Zinc Chloride Volume.
From pubs.acs.org
Zinc ChlorideCatalyzed Synthesis of Carbamates An Application for the Zinc Chloride Volume Write down the chemical formula of the compound. For example, water is h. [ weight to volume | volume to. Zinc chloride is an inorganic binary salt. The structure of a zncl 2 molecule is illustrated below. zinc chloride structure. zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble. Zinc Chloride Volume.