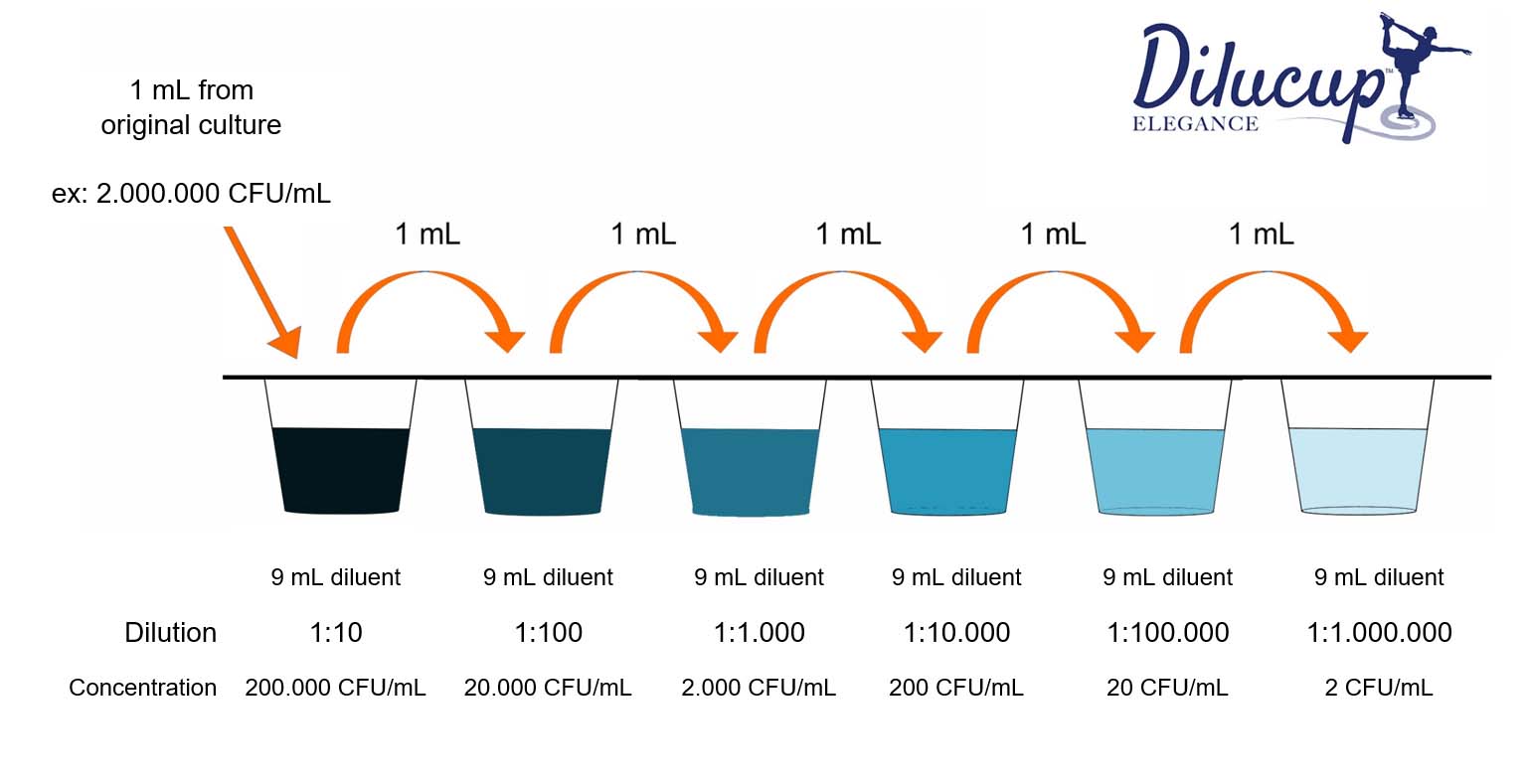
Dilution Up Meaning . Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. The dilution equation provides an. Concentration is the removal of solvent, which increases the concentration of. In order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: There are many ways of expressing concentration and dilution. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of.
from labrobot.com
In order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. There are many ways of expressing concentration and dilution. Concentration is the removal of. Explanations and examples of common methods. State whether the concentration of a solution is directly or indirectly proportional to its volume. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: The dilution equation provides an. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of.
Dilutions décimales Microbiologie alimentaire Système Dilucup
Dilution Up Meaning The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. There are many ways of expressing concentration and dilution. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: The dilution equation provides an. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of. Concentration is the removal of solvent, which increases the concentration of.
From bdaschools.weebly.com
bdaschools Blog Dilution Up Meaning In order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The action of making a liquid weaker by mixing in something else, or a liquid that. Dilution Up Meaning.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Up Meaning In order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Concentration is the removal of. Dilution is the addition. Dilution Up Meaning.
From joijqqtnu.blob.core.windows.net
Dilution Land Meaning at Bernice Blalock blog Dilution Up Meaning There are many ways of expressing concentration and dilution. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: The dilution equation provides an. Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State. Dilution Up Meaning.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilution Up Meaning Concentration is the removal of. The dilution equation provides an. Explanations and examples of common methods. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In. Dilution Up Meaning.
From joijqqtnu.blob.core.windows.net
Dilution Land Meaning at Bernice Blalock blog Dilution Up Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of. There are many ways of expressing concentration and dilution. Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute. Dilution Up Meaning.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilution Up Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. The dilution equation provides an. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Concentration is the removal of solvent, which increases the concentration. Dilution Up Meaning.
From www.slashdotblog.com
What Is Dilution? Dilution Up Meaning The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Concentration is the removal of. Explanations and examples of common methods. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the. Dilution Up Meaning.
From facts.net
13 Surprising Facts About Dilution Dilution Up Meaning Explanations and examples of common methods. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: There are many ways of expressing concentration and dilution. Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of. Dilution is the addition of solvent,. Dilution Up Meaning.
From www.fool.com
Understanding Stock Dilution and Why You Should Care About It The Dilution Up Meaning There are many ways of expressing concentration and dilution. Explanations and examples of common methods. The dilution equation provides an. In order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. The action of making a liquid weaker by mixing in something else,. Dilution Up Meaning.
From exytxbygt.blob.core.windows.net
Dilution Definition Literature at Mark Foster blog Dilution Up Meaning The dilution equation provides an. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of. Explanations and examples of common methods. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the concentration of. The action. Dilution Up Meaning.
From dxoeuuwhc.blob.core.windows.net
Dilution Calculator Idt at Jennifer Hansen blog Dilution Up Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. The dilution equation provides an. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Dilution is the addition of. Dilution Up Meaning.
From joigsqngn.blob.core.windows.net
Dilution Meaning Malayalam at Estella Steck blog Dilution Up Meaning The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In order to make a solution, we often take a. Dilution Up Meaning.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Up Meaning Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of. The dilution equation provides an. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. The. Dilution Up Meaning.
From www.youtube.com
150 dilution.why need this dilution?2 easy methods to preparation Dilution Up Meaning State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the concentration of. The dilution equation provides an. There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In order to make. Dilution Up Meaning.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Up Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Explanations and examples of common methods. Dilution is the addition. Dilution Up Meaning.
From joiubivum.blob.core.windows.net
How To Dilute Jeyes Fluid at John Contreras blog Dilution Up Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of. There are many ways of expressing concentration and dilution. Explanations and examples of common methods. Concentration is the removal of solvent, which increases. Dilution Up Meaning.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID Dilution Up Meaning There are many ways of expressing concentration and dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker. Dilution Up Meaning.
From joigsqngn.blob.core.windows.net
Dilution Meaning Malayalam at Estella Steck blog Dilution Up Meaning Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is. Dilution Up Meaning.
From extension.uga.edu
Understanding Laboratory Wastewater Tests I. ORGANICS (BOD, COD, TOC Dilution Up Meaning Concentration is the removal of solvent, which increases the concentration of. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: In order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the. Dilution Up Meaning.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Up Meaning The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. In order to make a solution,. Dilution Up Meaning.
From cemosaan.blob.core.windows.net
Dilution Added To Water at Larry Reis blog Dilution Up Meaning In order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The action of making a liquid weaker by mixing in something else, or a liquid that. Dilution Up Meaning.
From www.youtube.com
Dilution Meaning YouTube Dilution Up Meaning Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of. State whether the concentration of a solution is directly or indirectly proportional to its volume. The action of making a liquid weaker by mixing in something else, or a liquid that has been. Dilution Up Meaning.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube Dilution Up Meaning The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: In order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. The dilution equation provides an. The action of making. Dilution Up Meaning.
From exyveacqb.blob.core.windows.net
Serial Dilution Questions Pharmacy at Bruce Guerra blog Dilution Up Meaning Explanations and examples of common methods. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: State whether the concentration of a solution is directly or indirectly proportional to its volume. There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which. Dilution Up Meaning.
From www.youtube.com
How to prepare a Serial Dilution YouTube Dilution Up Meaning The dilution equation provides an. Concentration is the removal of. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of. Dilution Up Meaning.
From mmerevise.co.uk
Concentrations and Dilutions MME Dilution Up Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: There are many ways of. Dilution Up Meaning.
From ceysjldb.blob.core.windows.net
How To Graph Serial Dilutions at Linda Pike blog Dilution Up Meaning Concentration is the removal of solvent, which increases the concentration of. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: State whether the. Dilution Up Meaning.
From labrobot.com
Dilutions décimales Microbiologie alimentaire Système Dilucup Dilution Up Meaning There are many ways of expressing concentration and dilution. In order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases. Dilution Up Meaning.
From exytxbygt.blob.core.windows.net
Dilution Definition Literature at Mark Foster blog Dilution Up Meaning In order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. Concentration is the removal of solvent, which increases the concentration of. Concentration is the removal of. State whether the concentration of a solution is directly or indirectly proportional to its volume. The. Dilution Up Meaning.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilution Up Meaning The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Concentration is the removal of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent,. Dilution Up Meaning.
From exyveacqb.blob.core.windows.net
Serial Dilution Questions Pharmacy at Bruce Guerra blog Dilution Up Meaning State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way:. Dilution Up Meaning.
From dxoecpgvz.blob.core.windows.net
How To Dilutions at Christopher Stevenson blog Dilution Up Meaning The dilution equation provides an. There are many ways of expressing concentration and dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Concentration is the removal of solvent, which increases. Dilution Up Meaning.
From mungfali.com
10 Fold Serial Dilution Dilution Up Meaning The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: Explanations and examples of common methods. Concentration is the removal of. Concentration is the removal of solvent, which increases the concentration of. The dilution equation provides an. The action of making a liquid weaker by mixing in. Dilution Up Meaning.
From www.youtube.com
Dilutions YouTube Dilution Up Meaning The dilution equation provides an. There are many ways of expressing concentration and dilution. Explanations and examples of common methods. The action of making a liquid weaker by mixing in something else, or a liquid that has been made weaker in this way: State whether the concentration of a solution is directly or indirectly proportional to its volume. The action. Dilution Up Meaning.
From fr.thptnganamst.edu.vn
Découvrir 184+ imagen dilution formule fr.thptnganamst.edu.vn Dilution Up Meaning State whether the concentration of a solution is directly or indirectly proportional to its volume. In order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The. Dilution Up Meaning.