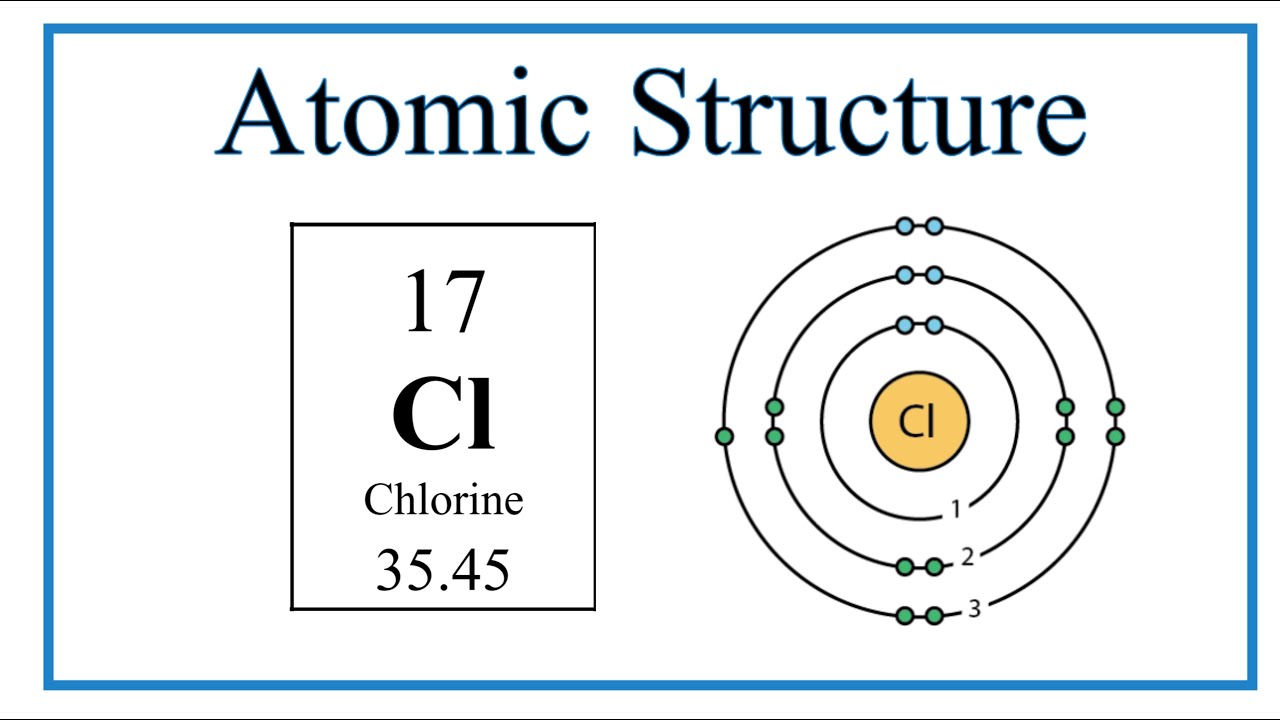
Chlorine Ion Structure . Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). The chloride ion is essential to life. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). It has a role as a human metabolite, an escherichia coli. As all halogens, it is thus one electron short of a full. It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. Chloride is a halide anion formed when chlorine picks up an electron to form an an anion.
from www.youtube.com
It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. As all halogens, it is thus one electron short of a full. Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). It has a role as a human metabolite, an escherichia coli. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. The chloride ion is essential to life. In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). Chloride is a halide anion formed when chlorine picks up an electron to form an an anion.
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube
Chlorine Ion Structure In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. The chloride ion is essential to life. In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. As all halogens, it is thus one electron short of a full. It has a role as a human metabolite, an escherichia coli. Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell.
From sciencenotes.org
Chlorine Facts Chlorine Ion Structure In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). As all halogens, it is thus one electron short of a full. The chloride ion is essential to life. It has a role as a human metabolite, an escherichia coli. Like fluorine and the other members of the halogen family, chlorine is diatomic. Chlorine Ion Structure.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Chlorine Ion Structure Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). The chloride ion is essential to life. It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. Chloride is a halide anion formed when chlorine picks up an electron to form. Chlorine Ion Structure.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Ion Structure In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). It has a role as a human metabolite, an escherichia coli. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. Chloride is a halide anion formed when chlorine picks up. Chlorine Ion Structure.
From www.dreamstime.com
An Atom of Chlorine Diagram Stock Vector Illustration of structure Chlorine Ion Structure Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)).. Chlorine Ion Structure.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Chlorine Ion Structure In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl.. Chlorine Ion Structure.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Ion Structure In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. It has a. Chlorine Ion Structure.
From dxopaibhs.blob.core.windows.net
A Chlorine Atom Gains An Electron. What Is The Resulting Particle at Chlorine Ion Structure It has a role as a human metabolite, an escherichia coli. It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. The chloride ion is essential to life. In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). As all halogens, it is thus one. Chlorine Ion Structure.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Ion Structure Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. The chloride ion is essential to life. As all halogens, it is thus one electron short of a full. It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. Chloride. Chlorine Ion Structure.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Chlorine Ion Structure Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). The chloride ion is essential to life. In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). It is mostly present in cell fluid as a negative ion to balance the positive. Chlorine Ion Structure.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Ion Structure Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). As all halogens, it is thus one electron short of a full. Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. It is mostly present in cell fluid as a negative. Chlorine Ion Structure.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C023/2480 Science Photo Chlorine Ion Structure Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). The chloride ion is essential to life. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons. Chlorine Ion Structure.
From www.alamy.com
Chlorine Element High Resolution Stock Photography and Images Alamy Chlorine Ion Structure In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. It has a. Chlorine Ion Structure.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Ion Structure In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). As all halogens, it is thus one electron short of a full. It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. Chloride also acts as a bridging ligand in which one, two or three. Chlorine Ion Structure.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Ion Structure It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. The chloride ion is essential to life. Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal. Chlorine Ion Structure.
From stock.adobe.com
Chlorine atomic structure has atomic number, atomic mass, electron Chlorine Ion Structure It has a role as a human metabolite, an escherichia coli. As all halogens, it is thus one electron short of a full. It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. Chlorine Ion Structure.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Ion Structure Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. The chloride ion is essential to life. It has a role as a human metabolite, an escherichia coli. Chlorine has the electron. Chlorine Ion Structure.
From brainly.in
Draw the atomic structure of a chlorine ion Brainly.in Chlorine Ion Structure Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. The chloride ion is essential to life. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence. Chlorine Ion Structure.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Ion Structure It has a role as a human metabolite, an escherichia coli. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). Like fluorine and the other members of. Chlorine Ion Structure.
From www.youtube.com
How to Draw the Lewis Dot Structure for Cl (Chloride ion) YouTube Chlorine Ion Structure The chloride ion is essential to life. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. As all halogens, it is thus one electron short of a full. Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure. Chlorine Ion Structure.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Ion Structure Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. It has a role as a human metabolite, an escherichia coli. The chloride ion is essential to life. Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. As all halogens,. Chlorine Ion Structure.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Ion Structure It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. The chloride ion is essential to life. It has a role as a human metabolite, an escherichia coli. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Chloride is a. Chlorine Ion Structure.
From mageechemistry11.blogspot.com
Chemistry 11 Bohr and Lewis electron dot diagrams Chlorine Ion Structure Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl.. Chlorine Ion Structure.
From wirelistetiquette.z13.web.core.windows.net
Chlorine Electron Dot Diagram Chlorine Ion Structure Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). As all halogens, it is thus one electron short of a full. The chloride ion is essential to life. It has a role as a human metabolite, an escherichia coli. It is mostly present in cell fluid as a. Chlorine Ion Structure.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Ion Structure It has a role as a human metabolite, an escherichia coli. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). As all halogens, it is thus one electron short of a. Chlorine Ion Structure.
From www.sciencephoto.com
Chlorine molecules Stock Image A602/0069 Science Photo Library Chlorine Ion Structure Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). It has a role as a human metabolite, an escherichia coli. Chloride is a halide anion formed when. Chlorine Ion Structure.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Chlorine Ion Structure It has a role as a human metabolite, an escherichia coli. The chloride ion is essential to life. As all halogens, it is thus one electron short of a full. It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. Chloride is a halide anion formed when chlorine picks up an electron. Chlorine Ion Structure.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Ion Structure In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. The chloride ion is essential to life. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\). Chlorine Ion Structure.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Ion Structure It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. The chloride ion is essential to life. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Chloride also acts as a bridging ligand in which one, two or three chlorides. Chlorine Ion Structure.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Ion Structure Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl.. Chlorine Ion Structure.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Ion Structure Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. It is mostly present in cell fluid as a negative ion to balance the positive (mainly potassium) ions. Chloride also acts. Chlorine Ion Structure.
From www.alamy.com
3d render of atom structure of chlorine isolated over white background Chlorine Ion Structure Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. The chloride ion is essential to life. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. As all halogens, it is thus one electron short of. Chlorine Ion Structure.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Ion Structure As all halogens, it is thus one electron short of a full. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather than cl. Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. Chloride also acts as a bridging ligand in which. Chlorine Ion Structure.
From mavink.com
Chlorine Molecule Diagram Chlorine Ion Structure Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (figure \(\pageindex{1}\)). Chloride is a halide anion formed when chlorine picks up an electron to form an an anion. It has a role as a human metabolite, an escherichia coli. As all halogens, it is thus one electron short of a. Chlorine Ion Structure.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Chlorine Ion Structure Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. The chloride ion is essential to life. As all halogens, it is thus one electron short of a full. Like fluorine and the other members of the halogen family, chlorine is diatomic in nature, occurring as \ (cl_2\) rather. Chlorine Ion Structure.
From ar.inspiredpencil.com
Chlorine Molecule Model Chlorine Ion Structure It has a role as a human metabolite, an escherichia coli. As all halogens, it is thus one electron short of a full. In this video we'll look at the atomic structure and bohr model for the chlorine atom (cl). Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost. Chlorine Ion Structure.