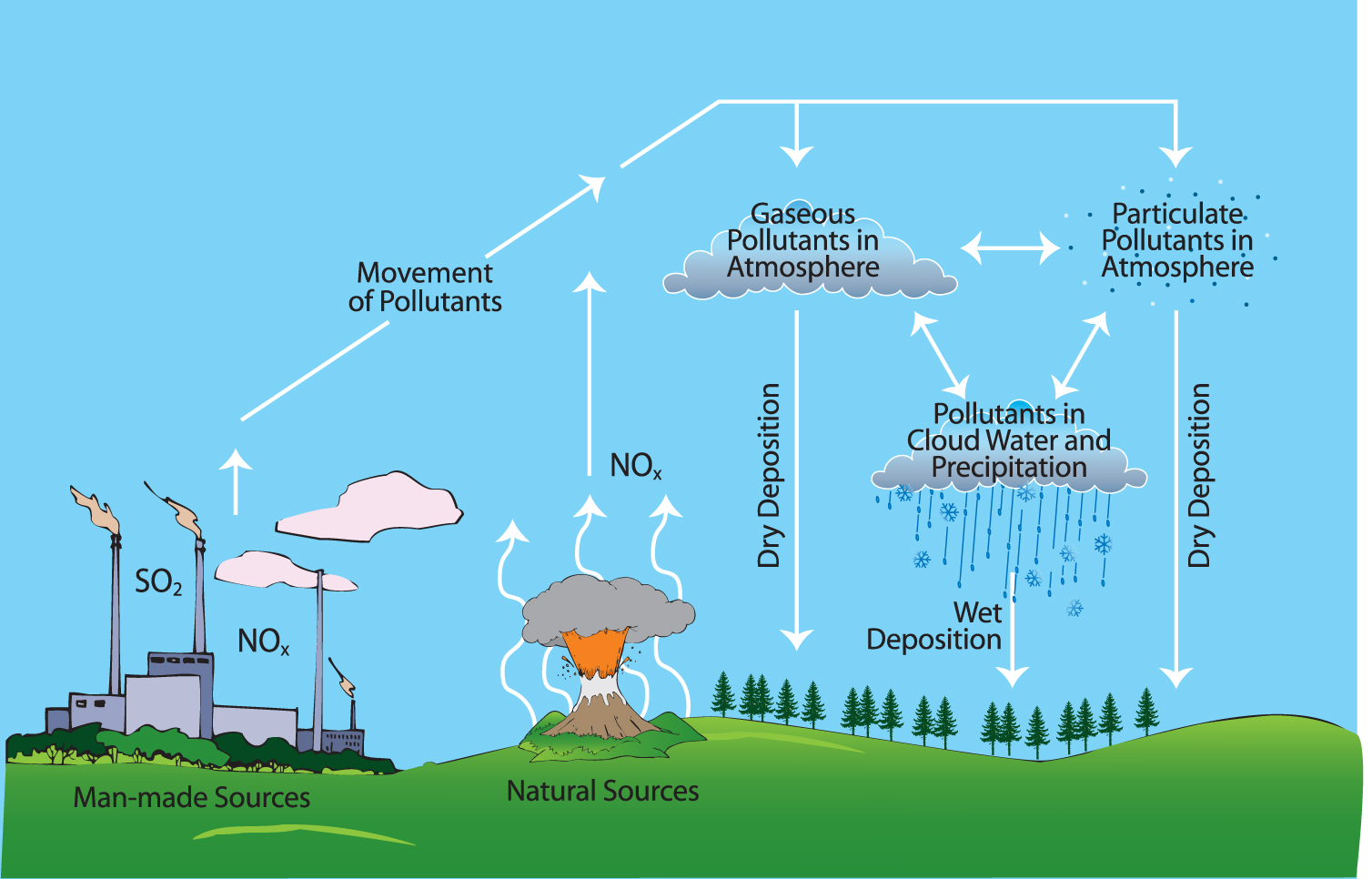
How Does Limestone React With Acid Rain . Marble and limestone both consist of calcium carbonate (caco 3), a salt. The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering. What happens in a reaction between acid rain and limestone? Because caso4 is somewhat soluble in water, significant. Limestone is mostly made up of the mineral calcium carbonate (caco3). When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This is not very soluble, so rocks don't dissolve very quickly. This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode.
from socratic.org
The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. What happens in a reaction between acid rain and limestone? Limestone is mostly made up of the mineral calcium carbonate (caco3). This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). Because caso4 is somewhat soluble in water, significant. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This is not very soluble, so rocks don't dissolve very quickly. This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years.
How does acid rain form? Socratic
How Does Limestone React With Acid Rain This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering. This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. What happens in a reaction between acid rain and limestone? When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This is not very soluble, so rocks don't dissolve very quickly. Limestone is mostly made up of the mineral calcium carbonate (caco3). Because caso4 is somewhat soluble in water, significant. Marble and limestone both consist of calcium carbonate (caco 3), a salt.
From www.scienceshorts.com
How Does Limestone Protect Lakes from Acid Rain How Does Limestone React With Acid Rain This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves.. How Does Limestone React With Acid Rain.
From socratic.org
How does acid rain form? Socratic How Does Limestone React With Acid Rain Limestone is mostly made up of the mineral calcium carbonate (caco3). This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering. This reaction occurs. How Does Limestone React With Acid Rain.
From fineartamerica.com
Limestone Reacting With Acid Photograph by Science Photo Library Fine Art America How Does Limestone React With Acid Rain When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. What happens in a reaction between acid rain and limestone? This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). This. How Does Limestone React With Acid Rain.
From www.scribd.com
Effect of Acid Rain On Limestone Rock PDF Limestone Chemistry How Does Limestone React With Acid Rain The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. This is not very soluble, so rocks don't dissolve very quickly. Because caso4 is somewhat soluble in water, significant. Limestone is mostly made up of the mineral calcium carbonate (caco3). The soluble substances in the acidic deposition get dissolved. How Does Limestone React With Acid Rain.
From www.alamy.com
Limestone acid reaction hires stock photography and images Alamy How Does Limestone React With Acid Rain When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This is not very soluble, so rocks don't dissolve very quickly. This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. Marble and limestone both consist of calcium carbonate. How Does Limestone React With Acid Rain.
From www.slideserve.com
PPT Weathering, Erosion, Deposition PowerPoint Presentation, free download ID373337 How Does Limestone React With Acid Rain What happens in a reaction between acid rain and limestone? When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). Because. How Does Limestone React With Acid Rain.
From www.slideserve.com
PPT Weathering and Soil Erosion PowerPoint Presentation, free download ID2452980 How Does Limestone React With Acid Rain Because caso4 is somewhat soluble in water, significant. This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). This is not very soluble, so rocks don't dissolve very quickly. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the. How Does Limestone React With Acid Rain.
From www.numerade.com
SOLVEDWrite an equation to show how sulfuric acids in acid rain reacts with marble and How Does Limestone React With Acid Rain This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. When sulfurous, sulfuric, and nitric acids in polluted air and rain. How Does Limestone React With Acid Rain.
From mavink.com
Acid Rain Limestone How Does Limestone React With Acid Rain When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Marble and limestone both consist of calcium carbonate (caco 3), a salt. This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. The soluble substances in the acidic deposition. How Does Limestone React With Acid Rain.
From www.scribd.com
Effect of Acid Rain On Limestone PDF Limestone Sulfuric Acid How Does Limestone React With Acid Rain When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and. How Does Limestone React With Acid Rain.
From mstimms-gcse.blogspot.com
Ms Timms GCSE Limestone reaction cycle chemistry How Does Limestone React With Acid Rain Limestone is mostly made up of the mineral calcium carbonate (caco3). This is not very soluble, so rocks don't dissolve very quickly. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone. How Does Limestone React With Acid Rain.
From www.slideserve.com
PPT Limestone Scenery PowerPoint Presentation, free download ID421349 How Does Limestone React With Acid Rain This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This reaction occurs very slowly and the effects are not normally. How Does Limestone React With Acid Rain.
From www.scienceshorts.com
How Does Limestone Protect Lakes from Acid Rain How Does Limestone React With Acid Rain The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. When. How Does Limestone React With Acid Rain.
From www.tes.com
Limestone Erosion and Acid Rain Teaching Resources How Does Limestone React With Acid Rain Marble and limestone both consist of calcium carbonate (caco 3), a salt. This is not very soluble, so rocks don't dissolve very quickly. Limestone is mostly made up of the mineral calcium carbonate (caco3). What happens in a reaction between acid rain and limestone? The soluble substances in the acidic deposition get dissolved in water and then washed away, known. How Does Limestone React With Acid Rain.
From www.slideserve.com
PPT Chemical Weathering of Rocks PowerPoint Presentation, free download ID1539022 How Does Limestone React With Acid Rain This is not very soluble, so rocks don't dissolve very quickly. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Limestone is mostly made up of the mineral calcium carbonate (caco3). This reaction occurs very slowly and the effects are not normally seen for hundreds or even. How Does Limestone React With Acid Rain.
From ian.umces.edu
Causes of acid rain Media Library Integration and Application Network How Does Limestone React With Acid Rain When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering.. How Does Limestone React With Acid Rain.
From www.sciencephoto.com
Limestone Reacts with Acid Stock Image C039/1326 Science Photo Library How Does Limestone React With Acid Rain Limestone is mostly made up of the mineral calcium carbonate (caco3). The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. What happens in a reaction between. How Does Limestone React With Acid Rain.
From www.internetgeography.net
How does weathering affect limestone? Geography How Does Limestone React With Acid Rain Limestone is mostly made up of the mineral calcium carbonate (caco3). This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. When sulfurous, sulfuric,. How Does Limestone React With Acid Rain.
From www.climateandweather.net
Acid Rain Climate & Weather How Does Limestone React With Acid Rain This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Limestone is mostly made up of the mineral calcium carbonate (caco3). What happens in a reaction between acid rain. How Does Limestone React With Acid Rain.
From www.scribd.com
Effect of Acid Rain On Limestone PDF How Does Limestone React With Acid Rain Limestone is mostly made up of the mineral calcium carbonate (caco3). This is not very soluble, so rocks don't dissolve very quickly. This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. What happens in a reaction between acid rain and limestone? The soluble substances in the acidic deposition get dissolved. How Does Limestone React With Acid Rain.
From www.slideserve.com
PPT Weathering, Erosion & Deposition PowerPoint Presentation ID4655832 How Does Limestone React With Acid Rain This is not very soluble, so rocks don't dissolve very quickly. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Because caso4 is somewhat soluble in water, significant. Limestone is mostly made up of the mineral calcium carbonate (caco3). This reaction occurs very slowly and the effects. How Does Limestone React With Acid Rain.
From www.sciencephoto.com
Reaction of limestone and acid Stock Image A500/0760 Science Photo Library How Does Limestone React With Acid Rain When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This is not very soluble, so rocks don't dissolve very quickly. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. What happens in a reaction between. How Does Limestone React With Acid Rain.
From www.youtube.com
Calcium carbonate reacts with hydrochloric acid. Limestone buildings in acid rain. YouTube How Does Limestone React With Acid Rain This is not very soluble, so rocks don't dissolve very quickly. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This neutralization reaction occurs naturally. How Does Limestone React With Acid Rain.
From www.scienceshorts.com
How Does Limestone Protect Lakes from Acid Rain How Does Limestone React With Acid Rain When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Because caso4 is somewhat soluble in water, significant. This reaction occurs very slowly and the effects. How Does Limestone React With Acid Rain.
From www.thenakedscientists.com
What happens when acid reacts with limestone? Science Questions How Does Limestone React With Acid Rain Limestone is mostly made up of the mineral calcium carbonate (caco3). When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This is not very soluble, so rocks don't dissolve very quickly. This reaction occurs very slowly and the effects are not normally seen for hundreds or even. How Does Limestone React With Acid Rain.
From fineartamerica.com
Limestone Reacting With Acid Photograph by Andrew Lambert Photography How Does Limestone React With Acid Rain Because caso4 is somewhat soluble in water, significant. Marble and limestone both consist of calcium carbonate (caco 3), a salt. What happens in a reaction between acid rain and limestone? When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. When sulfurous, sulfuric, and nitric acids in polluted. How Does Limestone React With Acid Rain.
From www.slideshare.net
034 acid rain erodes houses made of limestone How Does Limestone React With Acid Rain Marble and limestone both consist of calcium carbonate (caco 3), a salt. Limestone is mostly made up of the mineral calcium carbonate (caco3). When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. What happens in a reaction between acid rain and limestone? The soluble substances in the. How Does Limestone React With Acid Rain.
From www.youtube.com
REACTION OF SULPHURIC ACID AND LIMESTONE YouTube How Does Limestone React With Acid Rain Marble and limestone both consist of calcium carbonate (caco 3), a salt. This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite. How Does Limestone React With Acid Rain.
From users.highland.edu
The Chemistry of Acid Rain How Does Limestone React With Acid Rain Because caso4 is somewhat soluble in water, significant. This is not very soluble, so rocks don't dissolve very quickly. The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering. Marble and limestone both consist of calcium carbonate (caco 3), a salt. Limestone is mostly made up of the mineral calcium carbonate. How Does Limestone React With Acid Rain.
From www.numerade.com
SOLVEDWrite reactions to show how the nitric and sulfuric acids in acid rain react with marble How Does Limestone React With Acid Rain This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering. This reaction occurs very slowly and the effects are not normally seen for hundreds. How Does Limestone React With Acid Rain.
From fphoto.photoshelter.com
acid rain environment limestone atmospheric pollution Fundamental Photographs The Art of Science How Does Limestone React With Acid Rain The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering. This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves.. How Does Limestone React With Acid Rain.
From www.ehow.com
What Does Acid Rain Do to Limestone? ehow How Does Limestone React With Acid Rain This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This is not very soluble, so rocks don't dissolve very quickly. Limestone is mostly made up of the mineral. How Does Limestone React With Acid Rain.
From www.numerade.com
SOLVED Write an equation to show how sulfuric acids in acid rain reacts with marble and How Does Limestone React With Acid Rain The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. Because caso4 is somewhat soluble in water, significant. This neutralization reaction occurs naturally in the environment when weak acids in rain react with limestone and other rocks, resulting in erosion (the wearing away of rock). This reaction occurs very. How Does Limestone React With Acid Rain.
From stock.adobe.com
An image of acid rain impacting a limestone statue, with a chemical breakdown of the reaction How Does Limestone React With Acid Rain The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. Marble and limestone both consist of calcium carbonate (caco 3), a salt. This reaction occurs very slowly and the effects are not normally seen for hundreds or even thousands of years. When sulfurous, sulfuric, and nitric acids in polluted. How Does Limestone React With Acid Rain.
From www.sciencephoto.com
Limestone Reacting with HCl Stock Image A500/0783 Science Photo Library How Does Limestone React With Acid Rain The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering. Marble and limestone both consist of calcium carbonate (caco 3), a salt. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. When sulfurous, sulfuric, and nitric acids in polluted. How Does Limestone React With Acid Rain.