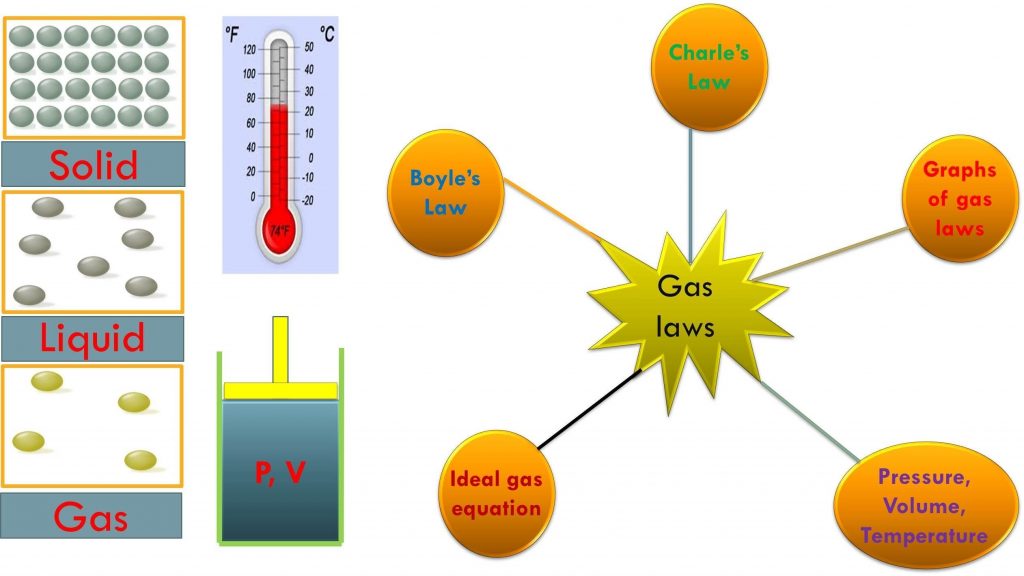
Chemistry Labs For Gas Laws . Gases have been one of the most useful types of substances for driving scientific discovery. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. three station gas lab. Determine the effect of pressure on volume of a gas. experiment 11 the gas laws. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. lansing community college general chemistry laboratory i. In this experiment you will (1) determine whether boyle’s law applies to a mixture.
from studiousguy.com
In this experiment you will (1) determine whether boyle’s law applies to a mixture. pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. experiment 11 the gas laws. lansing community college general chemistry laboratory i. three station gas lab. Determine the effect of pressure on volume of a gas. Gases have been one of the most useful types of substances for driving scientific discovery.
The Gas Laws Definition, Formula & Examples StudiousGuy
Chemistry Labs For Gas Laws Gases have been one of the most useful types of substances for driving scientific discovery. pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. In this experiment you will (1) determine whether boyle’s law applies to a mixture. Gases have been one of the most useful types of substances for driving scientific discovery. lansing community college general chemistry laboratory i. Determine the effect of pressure on volume of a gas. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. experiment 11 the gas laws. three station gas lab. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas.
From owlcation.com
The Theories and Behavior of Gas Owlcation Chemistry Labs For Gas Laws Gases have been one of the most useful types of substances for driving scientific discovery. Determine the effect of pressure on volume of a gas. three station gas lab. in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. there are three simple gas laws studying the various parameters like. Chemistry Labs For Gas Laws.
From printableniggie313l2.z22.web.core.windows.net
How To Calculate Gas Laws Chemistry Labs For Gas Laws experiment 11 the gas laws. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. Determine the effect of pressure on volume of a gas. Gases have been one of the most useful types of substances. Chemistry Labs For Gas Laws.
From www.studyxapp.com
chemistry ideal gas law constant introduction laboratory simulation a Chemistry Labs For Gas Laws in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. Determine the effect of pressure on volume of a gas. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. recognize the different gas laws such as boyle’s law,. Chemistry Labs For Gas Laws.
From obropolox.blogspot.com
40 chemistry gas laws worksheet with work Worksheet Resource Chemistry Labs For Gas Laws (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. Gases have been one of the most useful types of substances for driving scientific discovery. in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. there are three simple gas laws studying the various parameters like temperature (t), pressure (p),. Chemistry Labs For Gas Laws.
From www.youtube.com
Ideal Gas Law Lab YouTube Chemistry Labs For Gas Laws three station gas lab. Gases have been one of the most useful types of substances for driving scientific discovery. in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas.. Chemistry Labs For Gas Laws.
From studylib.net
AP CHEM NOTES GAS LAWS Chemistry Labs For Gas Laws Determine the effect of pressure on volume of a gas. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. lansing community college general chemistry laboratory i. three station gas lab. experiment 11 the gas laws. In this experiment you will (1) determine whether boyle’s law applies to a mixture. pump gas molecules to a. Chemistry Labs For Gas Laws.
From www.scribd.com
Chem Lab Report 9 (2)Gas Law Gases Temperature Chemistry Labs For Gas Laws in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. lansing community college general chemistry laboratory i. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. three station gas lab. there are. Chemistry Labs For Gas Laws.
From www.showme.com
Ideal gas law example Science, Chemistry, Gases ShowMe Chemistry Labs For Gas Laws in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. three station gas lab. lansing community college general chemistry laboratory i. experiment 11 the gas laws. . Chemistry Labs For Gas Laws.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Chemistry Labs For Gas Laws recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. lansing community college general chemistry laboratory i. pump gas molecules to a box and see what happens as you change the. Chemistry Labs For Gas Laws.
From chemistry101efhs.weebly.com
Gas Laws Chemistry 101 Chemistry Labs For Gas Laws experiment 11 the gas laws. In this experiment you will (1) determine whether boyle’s law applies to a mixture. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. in this simulation, students will investigate three of the fundamental gas laws, including. Chemistry Labs For Gas Laws.
From www.vernier.com
Exploring the Properties of Gases > Experiment 30 from Advanced Chemistry Labs For Gas Laws Gases have been one of the most useful types of substances for driving scientific discovery. experiment 11 the gas laws. Determine the effect of pressure on volume of a gas. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. three station gas lab. in this simulation, students will investigate three of. Chemistry Labs For Gas Laws.
From www.studocu.com
Gas Laws Chem 112 lab report Gas Law Warning TT undefined Chemistry Labs For Gas Laws (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. lansing community college general chemistry laboratory i. In this experiment you will (1) determine whether boyle’s law applies to a mixture. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. experiment 11 the gas laws. Gases have been one of the. Chemistry Labs For Gas Laws.
From maaphilross.blogspot.com
boyle's law experiment report Phil Ross Chemistry Labs For Gas Laws lansing community college general chemistry laboratory i. pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. Gases have been one of the most useful types of substances for driving scientific discovery. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. Determine the effect of. Chemistry Labs For Gas Laws.
From chemistry101efhs.weebly.com
Gas Laws Chemistry 101 Chemistry Labs For Gas Laws experiment 11 the gas laws. lansing community college general chemistry laboratory i. Gases have been one of the most useful types of substances for driving scientific discovery. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. Determine the effect of pressure on volume of a gas. pump gas molecules to a box and see what. Chemistry Labs For Gas Laws.
From mungfali.com
Gas Laws Cheat Sheet Chemistry Labs For Gas Laws Gases have been one of the most useful types of substances for driving scientific discovery. pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. there are three simple gas laws studying the various parameters like. Chemistry Labs For Gas Laws.
From studylib.net
Gas Laws Practice Test.Ans.Key Chemistry Labs For Gas Laws three station gas lab. in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. In this experiment you will (1) determine whether boyle’s law applies to a mixture. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. (81. Chemistry Labs For Gas Laws.
From www.youtube.com
Gas Laws Boyle's Law, Charles's Law, GayLussac’s Law and Avogadro's Chemistry Labs For Gas Laws In this experiment you will (1) determine whether boyle’s law applies to a mixture. pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. Determine the effect of pressure on volume of a. Chemistry Labs For Gas Laws.
From www.studocu.com
Lab 2 Gas Laws Chem lab Experiment 2 Experiment 2 (1 week) (GAS Chemistry Labs For Gas Laws In this experiment you will (1) determine whether boyle’s law applies to a mixture. experiment 11 the gas laws. Gases have been one of the most useful types of substances for driving scientific discovery. Determine the effect of pressure on volume of a gas. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws.. Chemistry Labs For Gas Laws.
From www.scribd.com
CHEM 1701 Lab 7 Gas Laws Chemistry I For PreHealth Sciences Chemistry Labs For Gas Laws in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. In this experiment you will (1) determine whether boyle’s law applies to a mixture. experiment 11 the gas laws. lansing community college general chemistry laboratory i. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. three station. Chemistry Labs For Gas Laws.
From www.youtube.com
How to Use Each Gas Law Study Chemistry With Us YouTube Chemistry Labs For Gas Laws experiment 11 the gas laws. Determine the effect of pressure on volume of a gas. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. Gases have been one of the most useful types of substances for driving scientific discovery. three station gas lab. pump. Chemistry Labs For Gas Laws.
From www.chegg.com
Gas Laws Lab Part 1 Collecting the gas in a balloon Chemistry Labs For Gas Laws Determine the effect of pressure on volume of a gas. three station gas lab. pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. experiment 11 the gas laws. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and. Chemistry Labs For Gas Laws.
From chem.libretexts.org
10 Experimental Determination of the Gas Constant (Experiment Chemistry Labs For Gas Laws experiment 11 the gas laws. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. Gases have been one of the most useful types of. Chemistry Labs For Gas Laws.
From oercommons.org
General Chemistry for Health Sciences lab manual 5 Gas laws OER Commons Chemistry Labs For Gas Laws there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. three station gas lab. pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. Gases have been one of the most useful types of substances. Chemistry Labs For Gas Laws.
From dxoyuouvw.blob.core.windows.net
Gas Laws Chemistry Khan Academy at Kasey Schenck blog Chemistry Labs For Gas Laws three station gas lab. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. experiment 11 the gas laws. Gases have been one of the most useful types of substances for. Chemistry Labs For Gas Laws.
From studiousguy.com
The Gas Laws Definition, Formula & Examples StudiousGuy Chemistry Labs For Gas Laws (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. Determine the effect of pressure on volume of a. Chemistry Labs For Gas Laws.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Chemistry Labs For Gas Laws three station gas lab. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. Determine the effect of pressure on volume of a gas. (81 favorites) lab in temperature,. Chemistry Labs For Gas Laws.
From www.youtube.com
Gas Law Formulas and Equations College Chemistry Study Guide YouTube Chemistry Labs For Gas Laws recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. in this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law. lansing community college general chemistry laboratory i. Determine the effect of pressure on volume of a gas. (81 favorites) lab in temperature, gas laws, pressure, volume,. Chemistry Labs For Gas Laws.
From in.pinterest.com
This sheet gives the formulas for the Gas Laws. MA.912.HSAAPR.C Chemistry Labs For Gas Laws Gases have been one of the most useful types of substances for driving scientific discovery. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. Determine the effect of pressure on volume of a gas. lansing community college general chemistry laboratory i. In this experiment you will. Chemistry Labs For Gas Laws.
From www.studypool.com
SOLUTION Lab 12 Gas Laws Studypool Chemistry Labs For Gas Laws there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. In this experiment you will (1) determine whether boyle’s law applies to a mixture. pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. Determine the. Chemistry Labs For Gas Laws.
From studylib.net
ideal gas law lab Chemistry Labs For Gas Laws In this experiment you will (1) determine whether boyle’s law applies to a mixture. three station gas lab. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. Determine the effect of pressure on volume of a gas. Gases have been one of. Chemistry Labs For Gas Laws.
From www.studocu.com
Gas laws assignment for GAS laws Chem 1010 Studocu Chemistry Labs For Gas Laws lansing community college general chemistry laboratory i. experiment 11 the gas laws. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. three station gas lab. Gases have been one of the most useful types of substances for driving scientific discovery. recognize the different. Chemistry Labs For Gas Laws.
From arbronsipva.weebly.com
Gaslawlabsforapchemistry REPACK Chemistry Labs For Gas Laws three station gas lab. Gases have been one of the most useful types of substances for driving scientific discovery. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. recognize the different gas laws such as boyle’s law, charles’s law and avogadro’s laws. in this simulation, students will investigate three of the fundamental gas laws, including. Chemistry Labs For Gas Laws.
From www.youtube.com
Gas Law Stoichiometry YouTube Chemistry Labs For Gas Laws pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. (81 favorites) lab in temperature, gas laws, pressure, volume, unlocked resources. Determine the effect of pressure on volume of a gas. lansing community college general chemistry laboratory i. In this experiment you will (1) determine whether boyle’s. Chemistry Labs For Gas Laws.
From sciencenotes.org
Ideal Gas Law Formula and Examples Chemistry Labs For Gas Laws Gases have been one of the most useful types of substances for driving scientific discovery. experiment 11 the gas laws. Determine the effect of pressure on volume of a gas. there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. (81 favorites) lab in temperature, gas laws,. Chemistry Labs For Gas Laws.
From www.chegg.com
Experiment VIII Gas Laws LABORATORY REPORT SHEET Date Chemistry Labs For Gas Laws there are three simple gas laws studying the various parameters like temperature (t), pressure (p), and number of moles of gas. pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. lansing community college general chemistry laboratory i. recognize the different gas laws such as. Chemistry Labs For Gas Laws.