Is A Candle An Open System . unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. we can also distinguish between open and closed systems: In an open system both matter and energy can enter or leave (we. a burning candle is an example of an open system because it exchanges both energy (in the form of. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. video of a scale over time as a candle burns in an open system. an open system is one in which energy can be transferred between the system and its surroundings.
from www.slideshare.net
video of a scale over time as a candle burns in an open system. In an open system both matter and energy can enter or leave (we. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. we can also distinguish between open and closed systems: an open system is one in which energy can be transferred between the system and its surroundings. unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. a burning candle is an example of an open system because it exchanges both energy (in the form of.
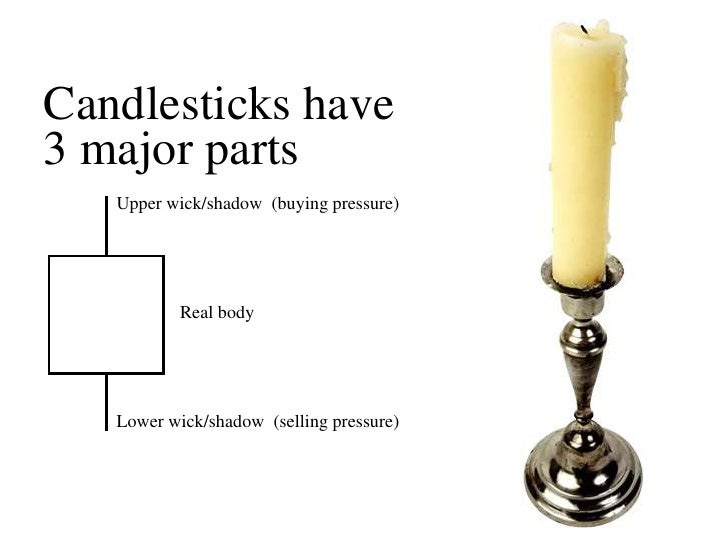
Candle presentation 3
Is A Candle An Open System within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. an open system is one in which energy can be transferred between the system and its surroundings. we can also distinguish between open and closed systems: a burning candle is an example of an open system because it exchanges both energy (in the form of. video of a scale over time as a candle burns in an open system. In an open system both matter and energy can enter or leave (we. unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree.
From www.theforexguy.com
An Introduction to the Power Candle Forex Trading Strategy Is A Candle An Open System we can also distinguish between open and closed systems: unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. In an. Is A Candle An Open System.
From www.youtube.com
What is better Open or Closed Candles YouTube Is A Candle An Open System we can also distinguish between open and closed systems: In an open system both matter and energy can enter or leave (we. an open system is one in which energy can be transferred between the system and its surroundings. unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. within. Is A Candle An Open System.
From www.youtube.com
How to Make Candles? Engineering Portal YouTube Is A Candle An Open System unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. an open system is one in which energy can be transferred between the system and its surroundings. In an open system both matter and energy can enter or leave (we. we can also distinguish between open and closed systems: video. Is A Candle An Open System.
From www.tradingfuel.com
10 Price Action Candlestick Patterns Trading Fuel Research Lab Is A Candle An Open System a burning candle is an example of an open system because it exchanges both energy (in the form of. an open system is one in which energy can be transferred between the system and its surroundings. unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. video of a scale. Is A Candle An Open System.
From officialbruinsshop.com
Candlestick Patterns Bruin Blog Is A Candle An Open System In an open system both matter and energy can enter or leave (we. we can also distinguish between open and closed systems: video of a scale over time as a candle burns in an open system. a burning candle is an example of an open system because it exchanges both energy (in the form of. within. Is A Candle An Open System.
From www.youtube.com
Candle Open High Low Close एक Candle कितने टाइम में बनता है? Is A Candle An Open System video of a scale over time as a candle burns in an open system. unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to. Is A Candle An Open System.
From www.istockphoto.com
Vector Illustration Of Candlestick Chart Components Composition Of A Is A Candle An Open System unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. a burning candle is an example of an open system because it exchanges both energy (in the form of. In an open system both matter and energy can enter or leave (we. video of a scale over time as a candle. Is A Candle An Open System.
From tradeciety.com
Mastering and Understanding Candlesticks Patterns Is A Candle An Open System a burning candle is an example of an open system because it exchanges both energy (in the form of. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. In an open system both matter and energy can. Is A Candle An Open System.
From www.ig.com
What is a Candlestick in Trading? IG UK Is A Candle An Open System video of a scale over time as a candle burns in an open system. a burning candle is an example of an open system because it exchanges both energy (in the form of. an open system is one in which energy can be transferred between the system and its surroundings. within science and the laws of. Is A Candle An Open System.
From kr.tradingview.com
VasilyTrader 의 BITSTAMPBTCUSD 용 How to Read a Candlestick Beginners Is A Candle An Open System we can also distinguish between open and closed systems: within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. an open system is one in which energy can be transferred between the system and its surroundings. . Is A Candle An Open System.
From outlookmoney.com
Understanding Candlesticks Charts Is A Candle An Open System In an open system both matter and energy can enter or leave (we. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. an open system is one in which energy can be transferred between the system and. Is A Candle An Open System.
From www.scienceabc.com
Science Of Candles How Do They Work? » Science ABC Is A Candle An Open System video of a scale over time as a candle burns in an open system. a burning candle is an example of an open system because it exchanges both energy (in the form of. unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. we can also distinguish between open and. Is A Candle An Open System.
From dotnettutorials.net
Mastering Candlestick Analysis in Trading Is A Candle An Open System within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. video of a scale over time as a candle burns in an open system. an open system is one in which energy can be transferred between the. Is A Candle An Open System.
From www.researchgate.net
Components in CANDLE with its Respective Modules Download Scientific Is A Candle An Open System unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. an open system is one in which energy can be transferred between the system and its surroundings. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of. Is A Candle An Open System.
From toughnickel.com
Stock Market Basics Candlestick Patterns ToughNickel Is A Candle An Open System we can also distinguish between open and closed systems: an open system is one in which energy can be transferred between the system and its surroundings. unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. In an open system both matter and energy can enter or leave (we. video. Is A Candle An Open System.
From www.brokerxplorer.com
Candle Breakout Strategy for Beginners Is A Candle An Open System we can also distinguish between open and closed systems: within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. video of a scale over time as a candle burns in an open system. In an open system. Is A Candle An Open System.
From tradingcryptocourse.com
5.5 Technical Analysis Candlesticks Trading Crypto Course Is A Candle An Open System In an open system both matter and energy can enter or leave (we. an open system is one in which energy can be transferred between the system and its surroundings. unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. video of a scale over time as a candle burns in. Is A Candle An Open System.
From www.pinterest.co.kr
Inside Day Candlestick Pattern The 50 Rule Day trading, Trading Is A Candle An Open System an open system is one in which energy can be transferred between the system and its surroundings. we can also distinguish between open and closed systems: video of a scale over time as a candle burns in an open system. a burning candle is an example of an open system because it exchanges both energy (in. Is A Candle An Open System.
From www.slideshare.net
Candle presentation 3 Is A Candle An Open System a burning candle is an example of an open system because it exchanges both energy (in the form of. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. video of a scale over time as a. Is A Candle An Open System.
From www.investopedia.com
Candlestick Chart Definition and Basics Explained Is A Candle An Open System a burning candle is an example of an open system because it exchanges both energy (in the form of. video of a scale over time as a candle burns in an open system. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of. Is A Candle An Open System.
From www.crowdspring.com
How to Start a Candle Business A StepbyStep Guide With Tips and Is A Candle An Open System a burning candle is an example of an open system because it exchanges both energy (in the form of. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. we can also distinguish between open and closed. Is A Candle An Open System.
From www.youtube.com
Trading Lesson Candlestick Formations YouTube Is A Candle An Open System an open system is one in which energy can be transferred between the system and its surroundings. we can also distinguish between open and closed systems: video of a scale over time as a candle burns in an open system. within science and the laws of thermodynamics, an open system is a system that allows both. Is A Candle An Open System.
From www.pinterest.com
Parts of a candlestick at Is A Candle An Open System unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. an open system is one in which energy can be transferred. Is A Candle An Open System.
From www.compellingtruth.org
What are the seven lampstands / candlesticks of Revelation? Is A Candle An Open System video of a scale over time as a candle burns in an open system. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. unlike closed systems, open systems exchange matter and energy with their surroundings, allowing. Is A Candle An Open System.
From www.youtube.com
Learn Forex Trading Candlestick Entry Techniques YouTube Is A Candle An Open System In an open system both matter and energy can enter or leave (we. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a. Is A Candle An Open System.
From inchemistry.acs.org
Shining a Light on Candles inChemistry Is A Candle An Open System we can also distinguish between open and closed systems: unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. a burning candle is an example of an open system because it exchanges both energy (in the form of. an open system is one in which energy can be transferred between. Is A Candle An Open System.
From dxodexxuu.blob.core.windows.net
Candles Explained at Janice Baker blog Is A Candle An Open System video of a scale over time as a candle burns in an open system. In an open system both matter and energy can enter or leave (we. an open system is one in which energy can be transferred between the system and its surroundings. a burning candle is an example of an open system because it exchanges. Is A Candle An Open System.
From www.forexstrategiesresources.com
The 3°Candle Scalping Trading System Forex Strategies Forex Is A Candle An Open System within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. a burning candle is an example of an open system because it exchanges both energy (in the form of. an open system is one in which energy. Is A Candle An Open System.
From www.btcc.com
16 Candlestick Patterns You Must Know and How to Read Them Is A Candle An Open System we can also distinguish between open and closed systems: unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of its boundaries to a significant degree. an. Is A Candle An Open System.
From www.investagrams.com
A Beginner’s Guide To Reading Candlestick Patterns InvestaDaily Is A Candle An Open System a burning candle is an example of an open system because it exchanges both energy (in the form of. video of a scale over time as a candle burns in an open system. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross the threshold of. Is A Candle An Open System.
From 2020edwardip.weebly.com
Edward's Science Awesomeness Candle Investigation Is A Candle An Open System In an open system both matter and energy can enter or leave (we. an open system is one in which energy can be transferred between the system and its surroundings. unlike closed systems, open systems exchange matter and energy with their surroundings, allowing for a continuous. video of a scale over time as a candle burns in. Is A Candle An Open System.
From www.investopedia.com
Understanding a Candlestick Chart Is A Candle An Open System a burning candle is an example of an open system because it exchanges both energy (in the form of. In an open system both matter and energy can enter or leave (we. we can also distinguish between open and closed systems: an open system is one in which energy can be transferred between the system and its. Is A Candle An Open System.
From vladimirribakov.com
Your Ultimate Guide to Trading with Heikin Ashi Candles Vladimir Ribakov Is A Candle An Open System an open system is one in which energy can be transferred between the system and its surroundings. a burning candle is an example of an open system because it exchanges both energy (in the form of. within science and the laws of thermodynamics, an open system is a system that allows both matter and energy to cross. Is A Candle An Open System.
From therobusttrader.com
Candlestick Guide How to Read Candlesticks and Chart Patterns Is A Candle An Open System video of a scale over time as a candle burns in an open system. a burning candle is an example of an open system because it exchanges both energy (in the form of. In an open system both matter and energy can enter or leave (we. we can also distinguish between open and closed systems: an. Is A Candle An Open System.
From dailytrademantra.com
What is Candlestick, Body of a candlestick, upper shadow & lower shadow Is A Candle An Open System a burning candle is an example of an open system because it exchanges both energy (in the form of. In an open system both matter and energy can enter or leave (we. an open system is one in which energy can be transferred between the system and its surroundings. we can also distinguish between open and closed. Is A Candle An Open System.