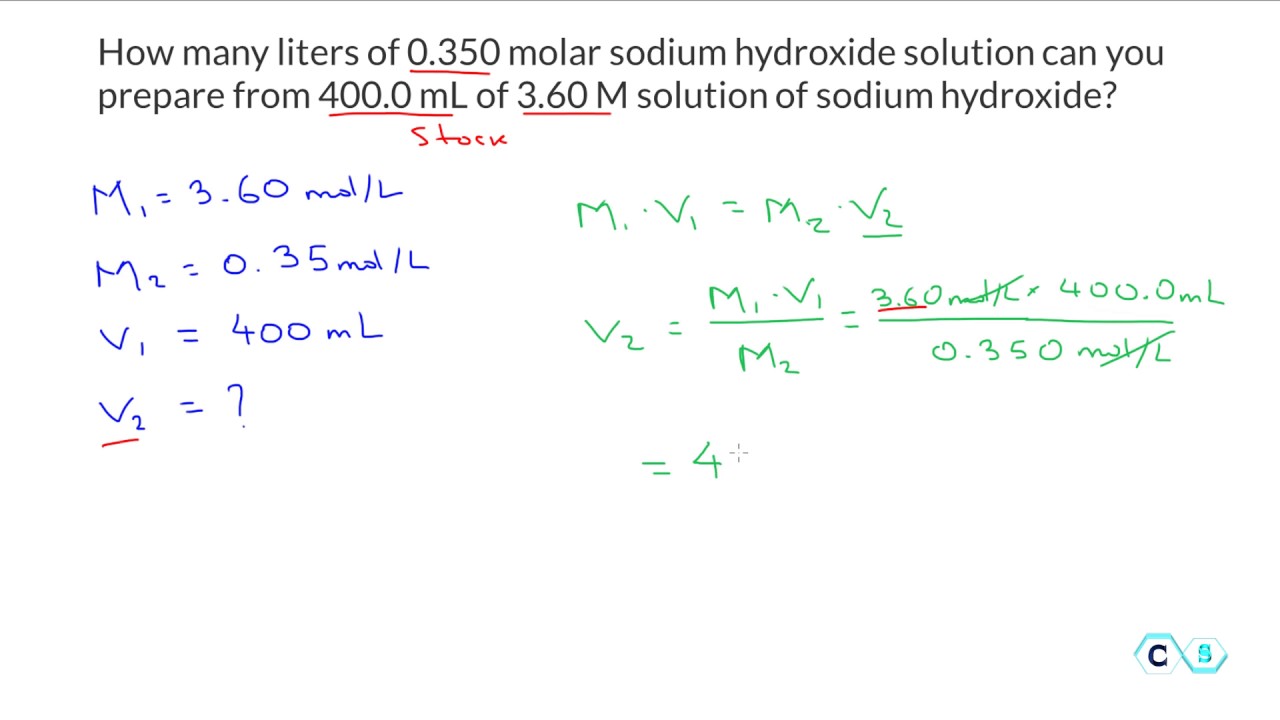
Molarity Dilution Practice Problems . 2) if i dilute 250 ml of 0.10 m. Moles solute before dilution = moles solute after dilution. Let us now write the definition of molarity: Study with quizlet and memorize. what is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? calculate the molarity of each of the following solutions: (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. M = moles of solute / volume of solution. For the first five problems, you need to use the equation that says that the molarity of. determine the molarity for each of the following solutions: 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 0.444 mol of cocl 2 in 0.654 l of solution. solutions to the molarity practice worksheet. I add 560 ml more water to it? please note how i use the molarity unit, mol/l, in the calculation rather than the molarity symbol, m.
from www.youtube.com
determine the molarity for each of the following solutions: For the first five problems, you need to use the equation that says that the molarity of. I add 560 ml more water to it? Study with quizlet and memorize. 2) if i dilute 250 ml of 0.10 m. Let us now write the definition of molarity: calculate the molarity of each of the following solutions: 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. what is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? 0.444 mol of cocl 2 in 0.654 l of solution.
Molarity Dilution Practice Problem 2 YouTube
Molarity Dilution Practice Problems Moles solute before dilution = moles solute after dilution. solutions to the molarity practice worksheet. I add 560 ml more water to it? For the first five problems, you need to use the equation that says that the molarity of. determine the molarity for each of the following solutions: calculate the molarity of each of the following solutions: 2) if i dilute 250 ml of 0.10 m. 0.444 mol of cocl 2 in 0.654 l of solution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Moles solute before dilution = moles solute after dilution. M = moles of solute / volume of solution. what is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? Study with quizlet and memorize. (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. Let us now write the definition of molarity: please note how i use the molarity unit, mol/l, in the calculation rather than the molarity symbol, m.
From www.chemistryspace.com
Molarity, Dilutions, and Titration • Practice Problems Molarity Dilution Practice Problems please note how i use the molarity unit, mol/l, in the calculation rather than the molarity symbol, m. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. M = moles of solute / volume of solution. (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of. Molarity Dilution Practice Problems.
From studyschoolnucleator.z21.web.core.windows.net
Molarity By Dilution Worksheet Molarity Dilution Practice Problems I add 560 ml more water to it? what is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? please note how i use the molarity unit, mol/l, in the calculation rather than the molarity symbol, m. Let us now write the definition of molarity:. Molarity Dilution Practice Problems.
From www.youtube.com
Molarity Practice Problems YouTube Molarity Dilution Practice Problems calculate the molarity of each of the following solutions: Moles solute before dilution = moles solute after dilution. M = moles of solute / volume of solution. (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. 2) if i dilute 250 ml of 0.10 m. I add 560 ml more water to. Molarity Dilution Practice Problems.
From lessonmagicwirtz.z13.web.core.windows.net
Molarity By Dilution Worksheets Molarity Dilution Practice Problems determine the molarity for each of the following solutions: Study with quizlet and memorize. M = moles of solute / volume of solution. Let us now write the definition of molarity: Moles solute before dilution = moles solute after dilution. calculate the molarity of each of the following solutions: 1) if i have 340 ml of a 0.5. Molarity Dilution Practice Problems.
From www.youtube.com
Gen Chem 1 Molarity and Dilution Practice Problems pt 1 YouTube Molarity Dilution Practice Problems 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Moles solute before dilution = moles solute after dilution. 0.444 mol of cocl 2 in 0.654 l of solution. I add 560 ml more water to it? solutions to the molarity practice worksheet. what is the required dilution factor for. Molarity Dilution Practice Problems.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Molarity Dilution Practice Problems 0.444 mol of cocl 2 in 0.654 l of solution. solutions to the molarity practice worksheet. Let us now write the definition of molarity: calculate the molarity of each of the following solutions: I add 560 ml more water to it? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be. Molarity Dilution Practice Problems.
From www.studocu.com
Practice Concentration Problems Calculate the Molarity of a solution Molarity Dilution Practice Problems I add 560 ml more water to it? M = moles of solute / volume of solution. please note how i use the molarity unit, mol/l, in the calculation rather than the molarity symbol, m. For the first five problems, you need to use the equation that says that the molarity of. 2) if i dilute 250 ml of. Molarity Dilution Practice Problems.
From www.worksheeto.com
7 Molarity Worksheet With Answers / Molarity Dilution Practice Problems Moles solute before dilution = moles solute after dilution. Study with quizlet and memorize. I add 560 ml more water to it? calculate the molarity of each of the following solutions: 2) if i dilute 250 ml of 0.10 m. what is the required dilution factor for each step, and how much of the original solution do you. Molarity Dilution Practice Problems.
From learningisidro.z13.web.core.windows.net
Making Dilutions Worksheets Molarity Dilution Practice Problems 2) if i dilute 250 ml of 0.10 m. Let us now write the definition of molarity: 0.444 mol of cocl 2 in 0.654 l of solution. please note how i use the molarity unit, mol/l, in the calculation rather than the molarity symbol, m. (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of. Molarity Dilution Practice Problems.
From www.youtube.com
WS Practice Problems Molarity and dilutions YouTube Molarity Dilution Practice Problems I add 560 ml more water to it? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. solutions to the molarity practice worksheet. Study with quizlet and memorize. 0.444 mol of cocl 2 in 0.654 l of solution. Moles solute before dilution = moles solute after dilution. determine the. Molarity Dilution Practice Problems.
From studylib.net
Molarity Practice Worksheet Molarity Dilution Practice Problems 0.444 mol of cocl 2 in 0.654 l of solution. I add 560 ml more water to it? For the first five problems, you need to use the equation that says that the molarity of. 2) if i dilute 250 ml of 0.10 m. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration. Molarity Dilution Practice Problems.
From www.youtube.com
Gen Chem 1 Molarity and Dilution Practice Problems pt 2 YouTube Molarity Dilution Practice Problems 0.444 mol of cocl 2 in 0.654 l of solution. Moles solute before dilution = moles solute after dilution. determine the molarity for each of the following solutions: For the first five problems, you need to use the equation that says that the molarity of. Let us now write the definition of molarity: please note how i use. Molarity Dilution Practice Problems.
From www.youtube.com
Molarity and Dilution Calculations YouTube Molarity Dilution Practice Problems please note how i use the molarity unit, mol/l, in the calculation rather than the molarity symbol, m. calculate the molarity of each of the following solutions: (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. Moles solute before dilution = moles solute after dilution. solutions to the molarity practice. Molarity Dilution Practice Problems.
From studycampusmatthews.z1.web.core.windows.net
Molarity Practice Problems Worksheets Molarity Dilution Practice Problems Let us now write the definition of molarity: 0.444 mol of cocl 2 in 0.654 l of solution. M = moles of solute / volume of solution. (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. what is the required dilution factor for each step, and how much of the original solution. Molarity Dilution Practice Problems.
From gersgiasbwa.blogspot.com
43 molarity practice worksheet answer Worksheet Master Molarity Dilution Practice Problems (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. M = moles of solute / volume of solution. 0.444 mol of cocl 2 in 0.654 l of solution. calculate the molarity of each of the following solutions: solutions to the molarity practice worksheet. what is the required dilution factor for. Molarity Dilution Practice Problems.
From lessonmagicpalladino.z13.web.core.windows.net
Molarity Practice Problems Worksheets Answers Molarity Dilution Practice Problems Let us now write the definition of molarity: Moles solute before dilution = moles solute after dilution. M = moles of solute / volume of solution. 0.444 mol of cocl 2 in 0.654 l of solution. determine the molarity for each of the following solutions: please note how i use the molarity unit, mol/l, in the calculation rather. Molarity Dilution Practice Problems.
From www.youtube.com
Molarity Dilution Practice Problem 2 YouTube Molarity Dilution Practice Problems calculate the molarity of each of the following solutions: determine the molarity for each of the following solutions: For the first five problems, you need to use the equation that says that the molarity of. M = moles of solute / volume of solution. 1) if i have 340 ml of a 0.5 m nabr solution, what will. Molarity Dilution Practice Problems.
From www.studocu.com
Dilutions volume change and molarity worksheet practice problems key Molarity Dilution Practice Problems Study with quizlet and memorize. (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. what is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? M = moles of solute / volume of solution. 2) if i dilute 250. Molarity Dilution Practice Problems.
From www.studocu.com
Molarity & Dilutions Practice Problems Molarity & Dilution Practice Molarity Dilution Practice Problems (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. For the first five problems, you need to use the equation that says that the molarity of. please note how i use the molarity unit, mol/l, in the calculation rather than the molarity symbol, m. 2) if i dilute 250 ml of 0.10. Molarity Dilution Practice Problems.
From www.docsity.com
Molarity and Dilutions Practice Worksheet Key Docsity Molarity Dilution Practice Problems M = moles of solute / volume of solution. solutions to the molarity practice worksheet. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. For the first five problems, you need to use the equation that says that the molarity of. 0.444 mol of cocl 2 in 0.654 l of. Molarity Dilution Practice Problems.
From studyschoolford.z21.web.core.windows.net
Molarity Dilution Practice Problems Molarity Dilution Practice Problems please note how i use the molarity unit, mol/l, in the calculation rather than the molarity symbol, m. what is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? determine the molarity for each of the following solutions: 2) if i dilute 250 ml. Molarity Dilution Practice Problems.
From www.studocu.com
Molarity & Dilutions Practice Problems Answers Molarity & Dilution Molarity Dilution Practice Problems 2) if i dilute 250 ml of 0.10 m. Moles solute before dilution = moles solute after dilution. Let us now write the definition of molarity: For the first five problems, you need to use the equation that says that the molarity of. solutions to the molarity practice worksheet. M = moles of solute / volume of solution. . Molarity Dilution Practice Problems.
From learninglibwegener.z1.web.core.windows.net
Molarity Practice Problems Worksheets Molarity Dilution Practice Problems determine the molarity for each of the following solutions: calculate the molarity of each of the following solutions: Let us now write the definition of molarity: 0.444 mol of cocl 2 in 0.654 l of solution. 2) if i dilute 250 ml of 0.10 m. (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l. Molarity Dilution Practice Problems.
From es.scribd.com
Molarity and Molality Practice Problems Concentración molar Solución Molarity Dilution Practice Problems please note how i use the molarity unit, mol/l, in the calculation rather than the molarity symbol, m. Moles solute before dilution = moles solute after dilution. Let us now write the definition of molarity: (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. I add 560 ml more water to it?. Molarity Dilution Practice Problems.
From studyschoolford.z21.web.core.windows.net
Molarity Dilution Practice Problems Molarity Dilution Practice Problems 2) if i dilute 250 ml of 0.10 m. what is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. please note how i use the molarity. Molarity Dilution Practice Problems.
From www.studocu.com
Stoichiometry Using Molarity Practice Problems Stoichiometry Using Molarity Dilution Practice Problems Moles solute before dilution = moles solute after dilution. M = moles of solute / volume of solution. what is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? I add 560 ml more water to it? For the first five problems, you need to use. Molarity Dilution Practice Problems.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Molarity Dilution Practice Problems M = moles of solute / volume of solution. 2) if i dilute 250 ml of 0.10 m. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Moles solute before dilution = moles solute after dilution. calculate the molarity of each of the following solutions: 0.444 mol of cocl 2. Molarity Dilution Practice Problems.
From studyzonetaoloblollies.z13.web.core.windows.net
Molarity And Dilution Worksheet Molarity Dilution Practice Problems what is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? For the first five problems, you need to use the equation that says that the molarity of. 0.444 mol of cocl 2 in 0.654 l of solution. solutions to the molarity practice worksheet. 2). Molarity Dilution Practice Problems.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Molarity Dilution Practice Problems solutions to the molarity practice worksheet. 2) if i dilute 250 ml of 0.10 m. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. determine the molarity for each of the following solutions: I add 560 ml more water to it? (a) 0.195 g of cholesterol, c 27 h. Molarity Dilution Practice Problems.
From www.youtube.com
Molarity and Dilution Practice Problems YouTube Molarity Dilution Practice Problems Let us now write the definition of molarity: I add 560 ml more water to it? calculate the molarity of each of the following solutions: (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. For the first five problems, you need to use the equation that says that the molarity of. 2). Molarity Dilution Practice Problems.
From www.youtube.com
Working out answers to Molarity Practice Problems 2 YouTube Molarity Dilution Practice Problems 2) if i dilute 250 ml of 0.10 m. I add 560 ml more water to it? solutions to the molarity practice worksheet. Let us now write the definition of molarity: what is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? determine the. Molarity Dilution Practice Problems.
From www.youtube.com
Molarity Practice Problems YouTube Molarity Dilution Practice Problems (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. 2) if i dilute 250 ml of 0.10 m. solutions to the molarity practice worksheet. M = moles of solute / volume of solution. Study with quizlet and memorize. I add 560 ml more water to it? calculate the molarity of each. Molarity Dilution Practice Problems.
From www.youtube.com
Practice Problems with Solutions, Concentration and Molarity YouTube Molarity Dilution Practice Problems I add 560 ml more water to it? solutions to the molarity practice worksheet. 2) if i dilute 250 ml of 0.10 m. (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. M = moles of solute / volume of solution. 0.444 mol of cocl 2 in 0.654 l of solution. . Molarity Dilution Practice Problems.
From printablelibswathed.z19.web.core.windows.net
Molarity And Dilution Worksheet Molarity Dilution Practice Problems determine the molarity for each of the following solutions: 0.444 mol of cocl 2 in 0.654 l of solution. what is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? Study with quizlet and memorize. 1) if i have 340 ml of a 0.5 m. Molarity Dilution Practice Problems.
From www.pinterest.com.au
Molarity Practice Problems Chemistry notes, Chemistry classroom Molarity Dilution Practice Problems (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the. M = moles of solute / volume of solution. Study with quizlet and memorize. what is the required dilution factor for each step, and how much of the original solution do you need to reach the final dilution? 2) if i dilute 250. Molarity Dilution Practice Problems.