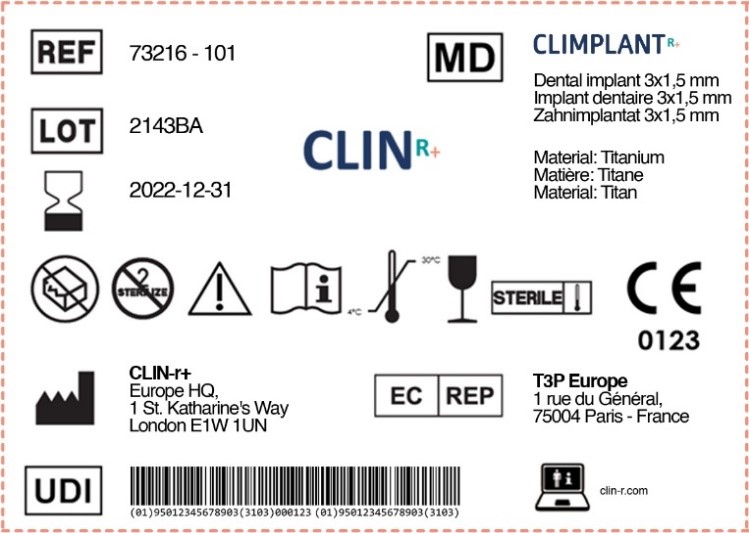
Medical Device Labelling Guidance . this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. specifically, this document provides guidance on the content of the label, instructions for use, and information. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical.
from clin-r.com
specifically, this document provides guidance on the content of the label, instructions for use, and information. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical.
Labels for Medical Devices Clin R
Medical Device Labelling Guidance specifically, this document provides guidance on the content of the label, instructions for use, and information. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device.
From mavink.com
Medical Device Labeling Symbols Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us. Medical Device Labelling Guidance.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Medical Device Labelling Guidance specifically, this document provides guidance on the content of the label, instructions for use, and information. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and. Medical Device Labelling Guidance.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what. Medical Device Labelling Guidance.
From www.schlafenderhase.com
A Guide to Medical Device Labeling Requirements Schlafender Hase Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of. Medical Device Labelling Guidance.
From www.morningtrans.com
International Medical Device Labeling Guide Morningside Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what. Medical Device Labelling Guidance.
From andamanmed.com
MDA/GD/0026 Labelling of Medical Devices Andaman Medical Medical Device Labelling Guidance this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. specifically, this document provides guidance on the content of the label, instructions for. Medical Device Labelling Guidance.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. this post will discuss what. Medical Device Labelling Guidance.
From hxelpllny.blob.core.windows.net
Medical Device Labeling Uk at Tomas Prather blog Medical Device Labelling Guidance specifically, this document provides guidance on the content of the label, instructions for use, and information. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what. Medical Device Labelling Guidance.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. this post will discuss what. Medical Device Labelling Guidance.
From dandelionsandthings.blogspot.com
30 Medical Device Label Symbols Label Design Ideas 2020 Medical Device Labelling Guidance •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of. Medical Device Labelling Guidance.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labelling Guidance this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. specifically, this document provides guidance on the content of the label, instructions for use, and information. •understand the requirements for medical device labeling •review some key labeling provisions for different. Medical Device Labelling Guidance.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Medical Device Labelling Guidance specifically, this document provides guidance on the content of the label, instructions for use, and information. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this post will discuss what. Medical Device Labelling Guidance.
From docslib.org
Guidance on Medical Device Patient Labeling; Final Guidance for Industry and FDA Reviewers DocsLib Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. specifically, this document provides guidance on the content of the label, instructions. Medical Device Labelling Guidance.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Registration & Labeling Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of. Medical Device Labelling Guidance.
From nextplus.io
Medical Device Labeling Compliant & UserFriendly Guide Next Plus Medical Device Labelling Guidance •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and. Medical Device Labelling Guidance.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Special Requirements RegDesk Medical Device Labelling Guidance •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. specifically, this document provides guidance on the content of the label, instructions for. Medical Device Labelling Guidance.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. •understand the requirements for medical device labeling •review some key labeling provisions. Medical Device Labelling Guidance.
From knobbemedical.com
Capture2 Knobbe Medical Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. specifically, this document provides guidance on the content of the label, instructions. Medical Device Labelling Guidance.
From compliancenavigator.bsigroup.com
Medical Device White Papers Medical Device Labelling Guidance specifically, this document provides guidance on the content of the label, instructions for use, and information. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. •understand the requirements for medical device labeling •review some key labeling provisions for different. Medical Device Labelling Guidance.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labelling Guidance specifically, this document provides guidance on the content of the label, instructions for use, and information. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this post will discuss what. Medical Device Labelling Guidance.
From datamyte.com
Medical Device Labeling A Comprehensive Guide DataMyte Medical Device Labelling Guidance •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this post will discuss what. Medical Device Labelling Guidance.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labelling Guidance •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. this guidance assists manufacturers in their development, and assist center reviewers in their. Medical Device Labelling Guidance.
From www.vrogue.co
Eu Mdr Medical Device Labeling Requirements A Complet vrogue.co Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us. Medical Device Labelling Guidance.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Labelling Guidance this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. specifically, this document provides guidance on the content of the label, instructions for use, and information. this guidance assists manufacturers in their development, and assist center reviewers in their review. Medical Device Labelling Guidance.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labelling Guidance specifically, this document provides guidance on the content of the label, instructions for use, and information. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. this guidance assists manufacturers in their development, and assist center reviewers in their review. Medical Device Labelling Guidance.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us. Medical Device Labelling Guidance.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Overview RegDesk Medical Device Labelling Guidance this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. specifically, this document provides guidance on the content of the label, instructions for use, and information. this guidance assists manufacturers in their development, and assist center reviewers in their review. Medical Device Labelling Guidance.
From www.scribd.com
Medical Device Labeling New ISO 152231 & FDA Guidance UDI Symbol Use PDF Medical Device Labelling Guidance •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and. Medical Device Labelling Guidance.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Symbol Use Medical Device Labelling Guidance specifically, this document provides guidance on the content of the label, instructions for use, and information. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and. Medical Device Labelling Guidance.
From www.pinterest.co.uk
Guidance Document Guidance for the Labelling of Medical Devices, not including in vitro Medical Device Labelling Guidance specifically, this document provides guidance on the content of the label, instructions for use, and information. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us. Medical Device Labelling Guidance.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Location and Content RegDesk Medical Device Labelling Guidance this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. specifically, this document provides guidance on the content of the label, instructions for use, and information. •understand the requirements for medical device labeling •review some key labeling provisions for different. Medical Device Labelling Guidance.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Labelling Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. •understand the requirements for medical device labeling •review some key labeling provisions. Medical Device Labelling Guidance.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Special Requirements RegDesk Medical Device Labelling Guidance this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. this guidance assists manufacturers in their development, and assist center reviewers in their. Medical Device Labelling Guidance.
From www.paladinid.com
Labeling Medical Devices A Guide PaladinID, LLC Label Solutions Medical Device Labelling Guidance this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. specifically, this document provides guidance on the content of the label, instructions for. Medical Device Labelling Guidance.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Medical Device Labelling Guidance •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and. Medical Device Labelling Guidance.