Bromine Trifluoride Fluoride . The chemical analysis of bromine trifluoride (brf3) was studied with the view to establishing a method for determining the purity of this. The physical and chemical properties of this substance are discussed below: Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. It is a hygroscopic liquid. The molar mass of bromine trifluoride is 136.90 g/mol. Bromine trifluoride is a fluoride of bromine. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. It is also a potent fluorinating agent and an ionizing inorganic solvent. The chemical has a pungent odor. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). The ability of bromine trifluoride in replacing halogens and even hydrogens with fluorine was the base for some very popular. Bromine trifluoride is used to produce uranium hexafluoride. It was discovered by paul lebeau in 1906.
from www.doubtnut.com
The physical and chemical properties of this substance are discussed below: The chemical has a pungent odor. Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). It is also a potent fluorinating agent and an ionizing inorganic solvent. It was discovered by paul lebeau in 1906. The ability of bromine trifluoride in replacing halogens and even hydrogens with fluorine was the base for some very popular. The molar mass of bromine trifluoride is 136.90 g/mol. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. The chemical analysis of bromine trifluoride (brf3) was studied with the view to establishing a method for determining the purity of this.
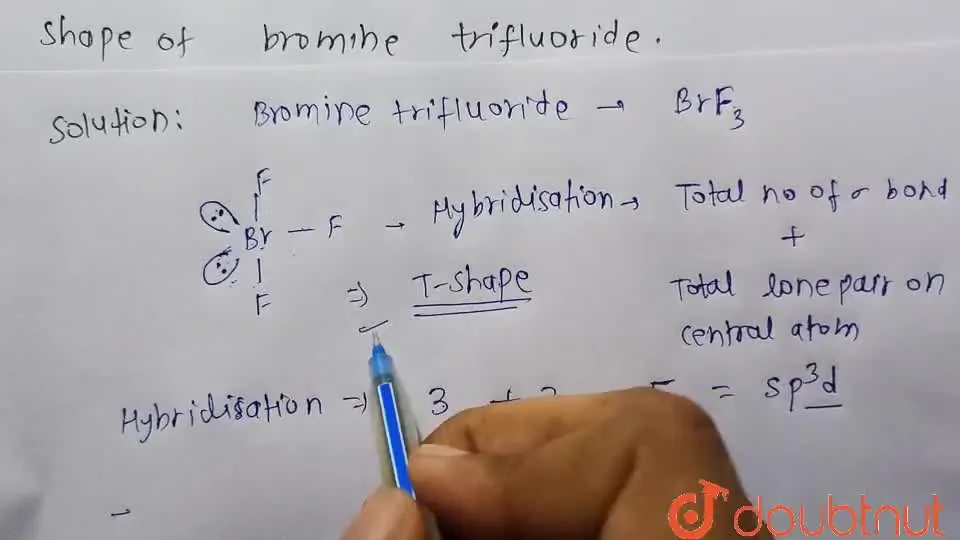
Draw structure and name the shape of bromine trifluoride
Bromine Trifluoride Fluoride The physical and chemical properties of this substance are discussed below: The molar mass of bromine trifluoride is 136.90 g/mol. It is also a potent fluorinating agent and an ionizing inorganic solvent. It was discovered by paul lebeau in 1906. The ability of bromine trifluoride in replacing halogens and even hydrogens with fluorine was the base for some very popular. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. It is a hygroscopic liquid. The chemical analysis of bromine trifluoride (brf3) was studied with the view to establishing a method for determining the purity of this. Bromine trifluoride is a fluoride of bromine. The physical and chemical properties of this substance are discussed below: Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. Bromine trifluoride is used to produce uranium hexafluoride. The chemical has a pungent odor.
From depositphotos.com
Bromine fluoride molecule — Stock Photo © jbouzou 1885785 Bromine Trifluoride Fluoride It is a hygroscopic liquid. The ability of bromine trifluoride in replacing halogens and even hydrogens with fluorine was the base for some very popular. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). Bromine trifluoride is used to produce uranium hexafluoride. Bromine trifluoride is. Bromine Trifluoride Fluoride.
From ar.inspiredpencil.com
Brf3 Molecule Bromine Trifluoride Fluoride Bromine trifluoride is a fluoride of bromine. The physical and chemical properties of this substance are discussed below: Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). Bromine trifluoride is used to produce uranium hexafluoride. Bromine trifluoride is a highly toxic and corrosive fluoride of. Bromine Trifluoride Fluoride.
From www.youtube.com
Is BrF3 (Bromine trifluoride) Ionic or Covalent/Molecular? YouTube Bromine Trifluoride Fluoride The chemical analysis of bromine trifluoride (brf3) was studied with the view to establishing a method for determining the purity of this. The chemical has a pungent odor. The molar mass of bromine trifluoride is 136.90 g/mol. Bromine trifluoride is used to produce uranium hexafluoride. It was discovered by paul lebeau in 1906. It is also a potent fluorinating agent. Bromine Trifluoride Fluoride.
From ar.inspiredpencil.com
Bromine Trifluoride Bromine Trifluoride Fluoride The molar mass of bromine trifluoride is 136.90 g/mol. The ability of bromine trifluoride in replacing halogens and even hydrogens with fluorine was the base for some very popular. It is also a potent fluorinating agent and an ionizing inorganic solvent. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. Bromine trifluoride is a strong fluorinating agent. Bromine Trifluoride Fluoride.
From www.ch.imperial.ac.uk
VSEPR Theory A closer look at bromine trifluoride, BrF3. Henry Rzepa Bromine Trifluoride Fluoride It was discovered by paul lebeau in 1906. It is also a potent fluorinating agent and an ionizing inorganic solvent. Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. The molar mass of bromine trifluoride is 136.90 g/mol. The physical and. Bromine Trifluoride Fluoride.
From exobvmtcw.blob.core.windows.net
Bromine Fluoride Structure at Guadalupe Lewis blog Bromine Trifluoride Fluoride Bromine trifluoride is used to produce uranium hexafluoride. The physical and chemical properties of this substance are discussed below: Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. It is a hygroscopic liquid. The chemical has a pungent odor. The chemical analysis of bromine trifluoride (brf3) was studied with the view to establishing a method for determining. Bromine Trifluoride Fluoride.
From www.smtgases.com
Bromine trifluoride, BrF3, Bromine Trifluoride Fluoride The physical and chemical properties of this substance are discussed below: It is also a potent fluorinating agent and an ionizing inorganic solvent. The molar mass of bromine trifluoride is 136.90 g/mol. It is a hygroscopic liquid. The ability of bromine trifluoride in replacing halogens and even hydrogens with fluorine was the base for some very popular. Bromine trifluoride is. Bromine Trifluoride Fluoride.
From chem.libretexts.org
10.5 Bromine Trifluoride as a Solvent Chemistry LibreTexts Bromine Trifluoride Fluoride It is a hygroscopic liquid. Bromine trifluoride is used to produce uranium hexafluoride. The molar mass of bromine trifluoride is 136.90 g/mol. Bromine trifluoride is a fluoride of bromine. The ability of bromine trifluoride in replacing halogens and even hydrogens with fluorine was the base for some very popular. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of. Bromine Trifluoride Fluoride.
From imgbin.com
Bromine Pentafluoride Bromine Trifluoride Lewis Structure Bromate PNG Bromine Trifluoride Fluoride Bromine trifluoride is a fluoride of bromine. The chemical has a pungent odor. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. It was discovered by paul lebeau in 1906. Bromine trifluoride is. Bromine Trifluoride Fluoride.
From www.anyrgb.com
Lithium Fluoride, lithium Nitride, bromine Trifluoride, Sodium alginate Bromine Trifluoride Fluoride Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. It is also a potent fluorinating agent and an ionizing inorganic solvent. It was discovered by paul lebeau in 1906. It is a hygroscopic liquid. Bromine trifluoride is a strong fluorinating agent. Bromine Trifluoride Fluoride.
From www.doubtnut.com
Draw structure and name the shape of bromine trifluoride Bromine Trifluoride Fluoride Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. The ability of bromine trifluoride in replacing halogens and even hydrogens with fluorine was the base for some very popular. Bromine trifluoride is a fluoride of bromine. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound,. Bromine Trifluoride Fluoride.
From www.youtube.com
Lewis Structure of BrF3 (bromine trifluoride) YouTube Bromine Trifluoride Fluoride It is a hygroscopic liquid. The molar mass of bromine trifluoride is 136.90 g/mol. Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. It is also a potent fluorinating agent and an ionizing inorganic solvent. The physical and chemical properties of this substance are discussed below: Bromine trifluoride is composed of 58.367% of. Bromine Trifluoride Fluoride.
From www.youtube.com
BrF3 Bromine Trifluoride Shape Hybridisation VSEPR Problem Bromine Trifluoride Fluoride The chemical analysis of bromine trifluoride (brf3) was studied with the view to establishing a method for determining the purity of this. Bromine trifluoride is used to produce uranium hexafluoride. Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. The ability of bromine trifluoride in replacing halogens and even hydrogens with fluorine was. Bromine Trifluoride Fluoride.
From ar.inspiredpencil.com
Bromine Trifluoride Bromine Trifluoride Fluoride It is a hygroscopic liquid. Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. Bromine trifluoride is used to produce uranium hexafluoride. The chemical analysis of bromine trifluoride (brf3) was studied with the view to establishing a method for determining the purity of this. It is also a potent fluorinating agent and an. Bromine Trifluoride Fluoride.
From exobvmtcw.blob.core.windows.net
Bromine Fluoride Structure at Guadalupe Lewis blog Bromine Trifluoride Fluoride Bromine trifluoride is a fluoride of bromine. It was discovered by paul lebeau in 1906. It is also a potent fluorinating agent and an ionizing inorganic solvent. The molar mass of bromine trifluoride is 136.90 g/mol. The chemical has a pungent odor. Bromine trifluoride is used to produce uranium hexafluoride. Bromine trifluoride is a highly toxic and corrosive fluoride of. Bromine Trifluoride Fluoride.
From wellcometreeoflife.org
BrF3 Molecular Geometry (2021) Everything You Need to Know Bromine Trifluoride Fluoride Bromine trifluoride is a fluoride of bromine. It was discovered by paul lebeau in 1906. The ability of bromine trifluoride in replacing halogens and even hydrogens with fluorine was the base for some very popular. The molar mass of bromine trifluoride is 136.90 g/mol. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium). Bromine Trifluoride Fluoride.
From ar.inspiredpencil.com
Bromine Trifluoride Bromine Trifluoride Fluoride The chemical has a pungent odor. It is a hygroscopic liquid. Bromine trifluoride is a fluoride of bromine. The molar mass of bromine trifluoride is 136.90 g/mol. The physical and chemical properties of this substance are discussed below: Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. Bromine trifluoride is composed of 58.367%. Bromine Trifluoride Fluoride.
From www.animalia-life.club
Brf3 Molecule Bromine Trifluoride Fluoride The chemical has a pungent odor. It was discovered by paul lebeau in 1906. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. It is also a potent fluorinating agent and an ionizing inorganic solvent. The molar mass of bromine trifluoride is 136.90 g/mol. Bromine trifluoride is a fluoride of bromine. Bromine trifluoride is used to produce. Bromine Trifluoride Fluoride.
From www.dreamstime.com
Bromine fluoride stock illustration. Illustration of molecular 8439119 Bromine Trifluoride Fluoride Bromine trifluoride is a fluoride of bromine. The molar mass of bromine trifluoride is 136.90 g/mol. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. The ability of bromine trifluoride in replacing halogens and even hydrogens with fluorine was the base for some very popular. The physical and chemical properties of this substance are discussed below: The. Bromine Trifluoride Fluoride.
From in.pinterest.com
Bromine trifluoride has the chemical formula BrF3 and interhalogen Bromine Trifluoride Fluoride The chemical has a pungent odor. Bromine trifluoride is a fluoride of bromine. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. The physical and chemical properties of this substance are discussed below: Bromine trifluoride is used to produce uranium hexafluoride. The chemical analysis of bromine trifluoride (brf3) was studied with the view to establishing a method. Bromine Trifluoride Fluoride.
From www.pinterest.com
BrF3 Molecular Geometry, Bond Angles(Bromine Trifluoride) Molecular Bromine Trifluoride Fluoride Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. It was discovered by paul lebeau in 1906. The chemical has a pungent odor. Bromine trifluoride is used to produce. Bromine Trifluoride Fluoride.
From ar.inspiredpencil.com
Bromine Trifluoride Bromine Trifluoride Fluoride The chemical analysis of bromine trifluoride (brf3) was studied with the view to establishing a method for determining the purity of this. Bromine trifluoride is used to produce uranium hexafluoride. It is also a potent fluorinating agent and an ionizing inorganic solvent. Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. Bromine trifluoride. Bromine Trifluoride Fluoride.
From imgbin.com
Interhalogen Bromine Pentafluoride Chlorine Trifluoride Iodine Bromine Trifluoride Fluoride It was discovered by paul lebeau in 1906. It is also a potent fluorinating agent and an ionizing inorganic solvent. It is a hygroscopic liquid. The molar mass of bromine trifluoride is 136.90 g/mol. Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. Bromine trifluoride is composed of 58.367% of bromine and 41.632%. Bromine Trifluoride Fluoride.
From dokumen.tips
(PDF) Selective Reactions of Bromine Trifluoride in Organic Chemistry Bromine Trifluoride Fluoride Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. The physical and chemical properties of this substance are discussed below: Bromine trifluoride is used to produce uranium hexafluoride. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). Bromine. Bromine Trifluoride Fluoride.
From favpng.com
Gold Fluoride Gold(III) Chloride Crystal Structure, PNG, 1024x1100px Bromine Trifluoride Fluoride Bromine trifluoride is a fluoride of bromine. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. It is a hygroscopic liquid. The molar mass of bromine trifluoride is 136.90 g/mol. Bromine trifluoride is used to produce uranium hexafluoride. The chemical analysis of bromine trifluoride (brf3) was studied with the view to establishing a method for determining the. Bromine Trifluoride Fluoride.
From www.dreamstime.com
Bromine fluoride molecule stock illustration. Illustration of bonding Bromine Trifluoride Fluoride It was discovered by paul lebeau in 1906. It is also a potent fluorinating agent and an ionizing inorganic solvent. The chemical has a pungent odor. The physical and chemical properties of this substance are discussed below: Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. The chemical analysis of bromine trifluoride (brf3). Bromine Trifluoride Fluoride.
From whatsinsight.org
BrF3 (Bromine trifluoride) Molecular Geometry, Bond Angles What's Insight Bromine Trifluoride Fluoride Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. It was discovered by paul lebeau in 1906. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). The molar mass of bromine trifluoride is 136.90 g/mol. The ability of bromine trifluoride in replacing. Bromine Trifluoride Fluoride.
From www.shapeways.com
Bromine Trifluoride (BrF3) (9SGE8EC22) by geoff_hutchison Bromine Trifluoride Fluoride Bromine trifluoride is used to produce uranium hexafluoride. It was discovered by paul lebeau in 1906. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. The physical and chemical properties of this substance are discussed below: The chemical has a pungent. Bromine Trifluoride Fluoride.
From www.chemtube3d.com
BrF3 Bromine trifluoride Bromine Trifluoride Fluoride Bromine trifluoride is a fluoride of bromine. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. The molar mass of bromine trifluoride is 136.90 g/mol. It is a hygroscopic liquid. Bromine trifluoride is used to produce uranium hexafluoride. It was discovered by paul lebeau in 1906. The chemical analysis of bromine trifluoride (brf3) was studied with the. Bromine Trifluoride Fluoride.
From chem.libretexts.org
10.5 Bromine Trifluoride as a Solvent Chemistry LibreTexts Bromine Trifluoride Fluoride Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. It is a hygroscopic liquid. Bromine trifluoride is a fluoride of bromine. The physical and chemical properties of this substance are discussed below: The chemical analysis of bromine trifluoride (brf3) was studied. Bromine Trifluoride Fluoride.
From imgbin.com
Chlorine Trifluoride Chlorine Pentafluoride Boron Trifluoride Chlorine Bromine Trifluoride Fluoride Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. It was discovered by paul lebeau in 1906. It is a hygroscopic liquid. The molar mass of bromine trifluoride is 136.90 g/mol. The chemical analysis of bromine trifluoride (brf3) was studied with the view to establishing a method for determining the purity of this. Bromine trifluoride is used. Bromine Trifluoride Fluoride.
From studylib.net
Bromine Trifluoride Bromine Trifluoride Fluoride Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. The physical and chemical properties of this substance are discussed below: It is a hygroscopic liquid. Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. The ability of bromine trifluoride in replacing halogens and even hydrogens with fluorine was the base. Bromine Trifluoride Fluoride.
From www.numerade.com
SOLVED According to the following reaction, how many grams of bromine Bromine Trifluoride Fluoride It is also a potent fluorinating agent and an ionizing inorganic solvent. It is a hygroscopic liquid. The chemical has a pungent odor. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. Bromine trifluoride is a strong fluorinating agent that is able to convert a metal (e.g., vanadium) to its associated fluoride compound, (i.e., vf 5). Bromine. Bromine Trifluoride Fluoride.
From www.alamy.com
BrF3 bromine trifluoride CAS 7787715 chemical substance in white Bromine Trifluoride Fluoride Bromine trifluoride is a highly toxic and corrosive fluoride of bromine with chemical formula brf 3. Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. It is also a potent fluorinating agent and an ionizing inorganic solvent. It is a hygroscopic liquid. Bromine trifluoride is a fluoride of bromine. The physical and chemical properties of this substance. Bromine Trifluoride Fluoride.
From whatsinsight.org
BrF3 (Bromine trifluoride) Molecular Geometry, Bond Angles What's Insight Bromine Trifluoride Fluoride Bromine trifluoride is composed of 58.367% of bromine and 41.632% of fluorine. Bromine trifluoride is used to produce uranium hexafluoride. It is a hygroscopic liquid. The chemical analysis of bromine trifluoride (brf3) was studied with the view to establishing a method for determining the purity of this. The chemical has a pungent odor. The physical and chemical properties of this. Bromine Trifluoride Fluoride.