Copper Ii Nitrate And Silver . Copper(ii) nitrate describes any member of the family of inorganic compounds with the formula cu(no 3) 2 (h 2 o) x. The balanced equation will appear. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and.
from www.numerade.com
One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. Copper(ii) nitrate describes any member of the family of inorganic compounds with the formula cu(no 3) 2 (h 2 o) x. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water.
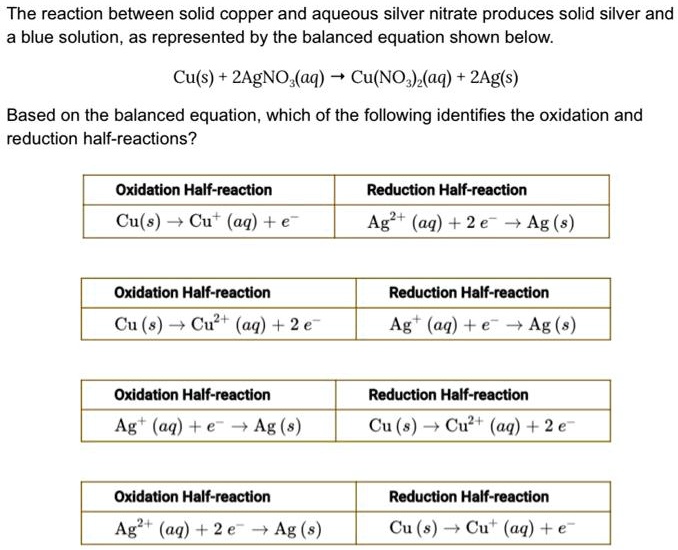
The reaction between solid copper and aqueous silver nitrate produces
Copper Ii Nitrate And Silver One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. Copper(ii) nitrate describes any member of the family of inorganic compounds with the formula cu(no 3) 2 (h 2 o) x. To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. The balanced equation will appear. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope.
From www.copperwiresuppliers.net
Copper Wire Silver Nitrate Copper Wire SuppliersCopper Wire Suppliers Copper Ii Nitrate And Silver One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes. Copper Ii Nitrate And Silver.
From www.alamy.com
Reaction of a rod of copper in a solution of silver nitrate.rs Stock Copper Ii Nitrate And Silver To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. The balanced equation will appear. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. Students discover that copper and tannic acids from tea. Copper Ii Nitrate And Silver.
From www.alamy.com
Copper coin being coated with silver crystals in a solution of silver Copper Ii Nitrate And Silver One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. The balanced equation will appear. One of the most fascinating chemical experiments is the. Copper Ii Nitrate And Silver.
From www.sciencephoto.com
Copper Reacts with Silver Nitrate, 4 of 6 Stock Image C028/1082 Copper Ii Nitrate And Silver To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Copper(ii) nitrate describes any member of the family of inorganic compounds with the formula cu(no 3) 2 (h 2 o) x. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. One of the most fascinating. Copper Ii Nitrate And Silver.
From www.numerade.com
SOLVED According to the following reaction, how many moles of copper Copper Ii Nitrate And Silver To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using. Copper Ii Nitrate And Silver.
From www.numerade.com
SOLVED2, When copper (LL) chloride reacts with silver nitrate, copper Copper Ii Nitrate And Silver Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. They complete the. Copper Ii Nitrate And Silver.
From www.chegg.com
Solved For the following reaction, 5.99 grams of silver Copper Ii Nitrate And Silver To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. Copper(ii) nitrate describes any member of the family of inorganic compounds with the formula cu(no 3) 2 (h 2 o) x. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of. Copper Ii Nitrate And Silver.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Ii Nitrate And Silver Copper(ii) nitrate describes any member of the family of inorganic compounds with the formula cu(no 3) 2 (h 2 o) x. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. To balance a chemical equation, enter an equation of a chemical reaction and press the balance. Copper Ii Nitrate And Silver.
From www.youtube.com
silver nitrate & copper time lapse YouTube Copper Ii Nitrate And Silver Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. The balanced equation will appear. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole. Copper Ii Nitrate And Silver.
From www.sciencephoto.com
Copper in Silver Nitrate Stock Image C002/7942 Science Photo Library Copper Ii Nitrate And Silver They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. Copper(ii) nitrate describes any member of the family of inorganic compounds with the formula cu(no 3) 2 (h 2 o) x. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form. Copper Ii Nitrate And Silver.
From www.sciencephoto.com
Copper reacting with silver nitrate Stock Image C030/8167 Science Copper Ii Nitrate And Silver One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. The balanced equation will appear. One mole of solid copper plus two moles of aqueous silver nitrate produce one. Copper Ii Nitrate And Silver.
From fphoto.photoshelter.com
science chemistry redox reaction silver nitrate copper Fundamental Copper Ii Nitrate And Silver One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. To balance. Copper Ii Nitrate And Silver.
From www.slideserve.com
PPT Preparation of silver nitrate and its uses PowerPoint Copper Ii Nitrate And Silver One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. The balanced. Copper Ii Nitrate And Silver.
From www.toppr.com
Vidisha placed a copper wire in silver nitrate solution as shown in the Copper Ii Nitrate And Silver To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Copper(ii) nitrate describes any member of the family of inorganic compounds with the formula cu(no 3) 2 (h 2 o) x. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. They complete the reaction, allow. Copper Ii Nitrate And Silver.
From www.wikiwand.com
Copper(II) nitrate Wikiwand Copper Ii Nitrate And Silver Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a. Copper Ii Nitrate And Silver.
From www.youtube.com
Copper and Silver Nitrate solution time lapse YouTube Copper Ii Nitrate And Silver Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. The balanced. Copper Ii Nitrate And Silver.
From lillyfersmcknight.blogspot.com
Copper Silver Nitrate Yields Copper Ii Nitrate Silver Copper Ii Nitrate And Silver One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. The balanced equation will appear. Students discover that copper and tannic acids from tea reduce silver nitrate, which in. Copper Ii Nitrate And Silver.
From www.alamy.com
Silver nitrate hires stock photography and images Alamy Copper Ii Nitrate And Silver To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using. Copper Ii Nitrate And Silver.
From www.slideserve.com
PPT Salts PowerPoint Presentation ID1487768 Copper Ii Nitrate And Silver To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using. Copper Ii Nitrate And Silver.
From www.sciencephoto.com
Copper Reacts with Silver Nitrate, 2 of 6 Stock Image C028/1080 Copper Ii Nitrate And Silver The balanced equation will appear. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Copper(ii) nitrate describes any member of the family of inorganic compounds with the formula cu(no. Copper Ii Nitrate And Silver.
From www.youtube.com
Copper And Silver Nitrate Make Silver And Copper (II) Nitrate YouTube Copper Ii Nitrate And Silver They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. Copper(ii) nitrate describes any member of the family of inorganic compounds with the formula. Copper Ii Nitrate And Silver.
From lunblogbrun.blogspot.com
Copper Metal and Aqueous Silver Nitrate LunBlogBrun Copper Ii Nitrate And Silver One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. Students discover that copper and tannic acids from tea reduce silver nitrate, which in. Copper Ii Nitrate And Silver.
From www.pinterest.com
Copper (II) Nitrate Trihydrate, 500g LabGrade The Curated Chemical Copper Ii Nitrate And Silver One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. Copper(ii) nitrate describes any member of the family of inorganic compounds with the formula cu(no 3) 2 (h 2 o) x. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles. Copper Ii Nitrate And Silver.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Ii Nitrate And Silver To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes. Copper Ii Nitrate And Silver.
From www.slideserve.com
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379 Copper Ii Nitrate And Silver To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag,. Copper Ii Nitrate And Silver.
From www.flinnsci.com
Flinn Chemicals, Copper(II) Nitrate Solution Copper Ii Nitrate And Silver The balanced equation will appear. One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. Students discover that copper and tannic acids from tea reduce silver nitrate, which in. Copper Ii Nitrate And Silver.
From uwaterloo.ca
Copper wire in a silver nitrate solution Chem 13 News Magazine Copper Ii Nitrate And Silver To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. Copper(ii) nitrate describes any member of the family of inorganic compounds with the formula cu(no 3) 2 (h 2 o) x. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form. Copper Ii Nitrate And Silver.
From shaunmwilliams.com
Lecture 5 Presentation Copper Ii Nitrate And Silver One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes. Copper Ii Nitrate And Silver.
From fphoto.photoshelter.com
displacement copper silver nitrate chemistry Fundamental Photographs Copper Ii Nitrate And Silver One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. The balanced equation will appear. One mole of solid copper plus two moles of aqueous silver nitrate produce one. Copper Ii Nitrate And Silver.
From www.numerade.com
The reaction between solid copper and aqueous silver nitrate produces Copper Ii Nitrate And Silver To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure to count all of ag, n, o, and. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. One of the most fascinating chemical experiments is the reaction between silver nitrate and. Copper Ii Nitrate And Silver.
From www.funcmater.com
wholesale Copper(II) nitrate hydrate Crystalline FUNCMATER Copper Ii Nitrate And Silver One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using. Copper Ii Nitrate And Silver.
From www.ravichemindustries.net
Copper Nitrate in Vadodara, India from Ravi Chem Industries Copper Ii Nitrate And Silver One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. To balance agno3 + cu → cu (no3)2. Copper Ii Nitrate And Silver.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Ii Nitrate And Silver The balanced equation will appear. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. To balance agno3 + cu → cu (no3)2 + ag you'll need to be sure. Copper Ii Nitrate And Silver.
From lillyfersmcknight.blogspot.com
Copper Silver Nitrate Yields Copper Ii Nitrate Silver Copper Ii Nitrate And Silver They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. The balanced equation will appear. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. One of the most fascinating chemical experiments is the. Copper Ii Nitrate And Silver.
From www.sciencephoto.com
Silver nitratecopper displacement reaction Stock Image C002/7894 Copper Ii Nitrate And Silver Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper(ii) nitrate plus two moles of solid. One of the most fascinating chemical experiments is the reaction between silver nitrate and copper wire in water. To balance. Copper Ii Nitrate And Silver.