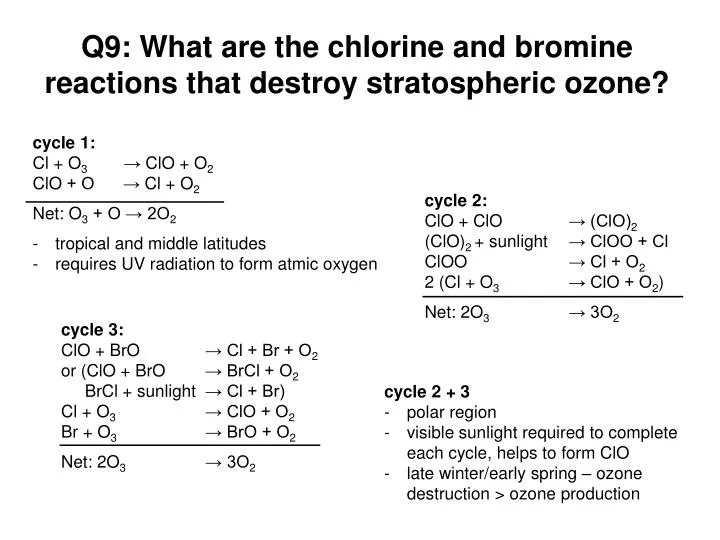
How Does Chlorine And Bromine Destroy Ozone . research studies in the laboratory show that chlorine (cl) reacts very rapidly with ozone. They also show that the reactive chemical chlorine. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated. chlorine, bromine, or iodine, is added to the stratosphere, the local ozone loss rates will generally increase, resulting in more. — these changes in atmospheric chemical composition suggest that wildfire aerosols affect stratospheric chlorine and. — when chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. learn how chlorine atoms catalytically destroy ozone molecules in the stratosphere, and how cfcs and other gases are involved in ozone depletion. the tropospheric equivalent chlorine (ec) abundance is the starting point for calculating the stratospheric halogen. — learn how chlorine and bromine radicals catalytically destroy ozone molecules in the stratosphere, leading to. each chlorine atom would react immediately with an ozone molecule, setting off a chain reaction that would destroy thousands of.
from www.slideserve.com
reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. each chlorine atom would react immediately with an ozone molecule, setting off a chain reaction that would destroy thousands of. — these changes in atmospheric chemical composition suggest that wildfire aerosols affect stratospheric chlorine and. chlorine, bromine, or iodine, is added to the stratosphere, the local ozone loss rates will generally increase, resulting in more. learn how chlorine atoms catalytically destroy ozone molecules in the stratosphere, and how cfcs and other gases are involved in ozone depletion. the tropospheric equivalent chlorine (ec) abundance is the starting point for calculating the stratospheric halogen. They also show that the reactive chemical chlorine. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. — when chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules.
PPT Q9 What are the chlorine and bromine reactions that destroy
How Does Chlorine And Bromine Destroy Ozone learn how chlorine atoms catalytically destroy ozone molecules in the stratosphere, and how cfcs and other gases are involved in ozone depletion. They also show that the reactive chemical chlorine. — chlorine acts as a catalyst in turning ozone into oxygen in a reaction that wasn't fully understood until 1973. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. research studies in the laboratory show that chlorine (cl) reacts very rapidly with ozone. — learn how chlorine and bromine radicals catalytically destroy ozone molecules in the stratosphere, leading to. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated. learn how chlorine atoms catalytically destroy ozone molecules in the stratosphere, and how cfcs and other gases are involved in ozone depletion. chlorine, bromine, or iodine, is added to the stratosphere, the local ozone loss rates will generally increase, resulting in more. — these changes in atmospheric chemical composition suggest that wildfire aerosols affect stratospheric chlorine and. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. — when chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules. each chlorine atom would react immediately with an ozone molecule, setting off a chain reaction that would destroy thousands of. bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction.
From slideplayer.com
Overview of air pollution ppt download How Does Chlorine And Bromine Destroy Ozone research studies in the laboratory show that chlorine (cl) reacts very rapidly with ozone. — chlorine acts as a catalyst in turning ozone into oxygen in a reaction that wasn't fully understood until 1973. — learn how chlorine and bromine radicals catalytically destroy ozone molecules in the stratosphere, leading to. chlorine, bromine, or iodine, is added. How Does Chlorine And Bromine Destroy Ozone.
From www.numerade.com
SOLVEDWhy does bromine destroy ozone more efficiently than does chlorine? How Does Chlorine And Bromine Destroy Ozone — these changes in atmospheric chemical composition suggest that wildfire aerosols affect stratospheric chlorine and. They also show that the reactive chemical chlorine. numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated. bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction. the tropospheric equivalent. How Does Chlorine And Bromine Destroy Ozone.
From www.ozonedisinfection.com.au
How Ozone Works Ozone Disinfection How Does Chlorine And Bromine Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. learn how chlorine atoms catalytically destroy ozone molecules in the stratosphere, and how cfcs and other gases are involved in ozone depletion. — these changes in atmospheric chemical composition suggest that wildfire aerosols affect stratospheric chlorine and. reactive. How Does Chlorine And Bromine Destroy Ozone.
From www.slideserve.com
PPT Q9 What are the chlorine and bromine reactions that destroy How Does Chlorine And Bromine Destroy Ozone — learn how chlorine and bromine radicals catalytically destroy ozone molecules in the stratosphere, leading to. learn how chlorine atoms catalytically destroy ozone molecules in the stratosphere, and how cfcs and other gases are involved in ozone depletion. — these changes in atmospheric chemical composition suggest that wildfire aerosols affect stratospheric chlorine and. chlorine, bromine, or. How Does Chlorine And Bromine Destroy Ozone.
From www.researchgate.net
Vertical profile of ozone concentration with the indication of the How Does Chlorine And Bromine Destroy Ozone chlorine, bromine, or iodine, is added to the stratosphere, the local ozone loss rates will generally increase, resulting in more. bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction. — chlorine acts as a catalyst in turning ozone into oxygen in a reaction that wasn't fully understood until 1973. . How Does Chlorine And Bromine Destroy Ozone.
From www.vrogue.co
Ozone Layer Depletion Greenhouse Effect Global Warmin vrogue.co How Does Chlorine And Bromine Destroy Ozone learn how chlorine atoms catalytically destroy ozone molecules in the stratosphere, and how cfcs and other gases are involved in ozone depletion. — when chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules. numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated. each chlorine. How Does Chlorine And Bromine Destroy Ozone.
From www.youtube.com
Simply Mechanisms 13b. Role of radicals in ozone formation and How Does Chlorine And Bromine Destroy Ozone each chlorine atom would react immediately with an ozone molecule, setting off a chain reaction that would destroy thousands of. bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction. learn how chlorine atoms catalytically destroy ozone molecules in the stratosphere, and how cfcs and other gases are involved in ozone. How Does Chlorine And Bromine Destroy Ozone.
From slideplayer.com
OBJECTIVE 3 HOW DOES HUMAN ACTIVITY ADVERSELY AFFECT THE ATMOSPHERE How Does Chlorine And Bromine Destroy Ozone — these changes in atmospheric chemical composition suggest that wildfire aerosols affect stratospheric chlorine and. bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. reactive gases containing chlorine and bromine destroy. How Does Chlorine And Bromine Destroy Ozone.
From www.researchgate.net
Simplified scheme of bromine explosion and ozone destruction reactions How Does Chlorine And Bromine Destroy Ozone research studies in the laboratory show that chlorine (cl) reacts very rapidly with ozone. They also show that the reactive chemical chlorine. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or. How Does Chlorine And Bromine Destroy Ozone.
From slideplayer.com
Climate Change and Ozone Depletion ppt download How Does Chlorine And Bromine Destroy Ozone the tropospheric equivalent chlorine (ec) abundance is the starting point for calculating the stratospheric halogen. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. — these changes in atmospheric. How Does Chlorine And Bromine Destroy Ozone.
From www.numerade.com
⏩SOLVEDWhy does bromine destroy ozone more efficiently than does How Does Chlorine And Bromine Destroy Ozone each chlorine atom would react immediately with an ozone molecule, setting off a chain reaction that would destroy thousands of. the tropospheric equivalent chlorine (ec) abundance is the starting point for calculating the stratospheric halogen. They also show that the reactive chemical chlorine. — learn how chlorine and bromine radicals catalytically destroy ozone molecules in the stratosphere,. How Does Chlorine And Bromine Destroy Ozone.
From csl.noaa.gov
NOAA CSL Scientific Assessment of Ozone Depletion 2014 How Does Chlorine And Bromine Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. — chlorine acts as a catalyst in turning ozone into oxygen in a reaction that wasn't fully understood until 1973. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. . How Does Chlorine And Bromine Destroy Ozone.
From csl.noaa.gov
NOAA CSL Scientific Assessment of Ozone Depletion 2002 How Does Chlorine And Bromine Destroy Ozone bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. chlorine, bromine, or iodine, is added to the stratosphere, the local ozone loss rates will generally increase, resulting in more. — chlorine. How Does Chlorine And Bromine Destroy Ozone.
From pdfprof.com
Q9 What are the chlorine and bromine reactions that destroy PDF How Does Chlorine And Bromine Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. the tropospheric equivalent chlorine (ec) abundance is the starting point for calculating the stratospheric halogen. numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated. bromine destroys ozone 50 times more efficiently than chlorine,. How Does Chlorine And Bromine Destroy Ozone.
From sciencing.com
How Does Chlorine Affect the Ozone Layer? Sciencing How Does Chlorine And Bromine Destroy Ozone chlorine, bromine, or iodine, is added to the stratosphere, the local ozone loss rates will generally increase, resulting in more. each chlorine atom would react immediately with an ozone molecule, setting off a chain reaction that would destroy thousands of. numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated. reactive gases. How Does Chlorine And Bromine Destroy Ozone.
From bio1151b.nicerweb.net
cfc.html 54_27ChlorineOzone.jpg How Does Chlorine And Bromine Destroy Ozone the tropospheric equivalent chlorine (ec) abundance is the starting point for calculating the stratospheric halogen. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. bromine destroys ozone 50 times. How Does Chlorine And Bromine Destroy Ozone.
From slideplayer.com
Air Pollution and Stratospheric Ozone Depletion ppt download How Does Chlorine And Bromine Destroy Ozone bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction. — these changes in atmospheric chemical composition suggest that wildfire aerosols affect stratospheric chlorine and. chlorine, bromine, or iodine, is added to the stratosphere, the local ozone loss rates will generally increase, resulting in more. learn how chlorine atoms catalytically. How Does Chlorine And Bromine Destroy Ozone.
From www.albany.edu
Ozone How Does Chlorine And Bromine Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. each chlorine atom would react immediately with an ozone molecule, setting off a chain reaction that would destroy thousands of. numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated. — learn how chlorine. How Does Chlorine And Bromine Destroy Ozone.
From www.slideserve.com
PPT Ozone Layer Depletion PowerPoint Presentation ID5461129 How Does Chlorine And Bromine Destroy Ozone — when chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules. numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. reactive gases containing chlorine and bromine. How Does Chlorine And Bromine Destroy Ozone.
From www.worldatlas.com
What Is The Ozone Hole? WorldAtlas How Does Chlorine And Bromine Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. — learn how chlorine and bromine radicals catalytically destroy ozone molecules in the stratosphere, leading to. bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction. reactive gases containing chlorine and bromine. How Does Chlorine And Bromine Destroy Ozone.
From www.csmonitor.com
Storm clouds could destroy ozone layer, study suggests How Does Chlorine And Bromine Destroy Ozone — these changes in atmospheric chemical composition suggest that wildfire aerosols affect stratospheric chlorine and. numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. each chlorine atom would react immediately with an ozone. How Does Chlorine And Bromine Destroy Ozone.
From slideplayer.com
The Ozone layer is changing ppt download How Does Chlorine And Bromine Destroy Ozone numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated. — when chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. the tropospheric equivalent chlorine (ec) abundance. How Does Chlorine And Bromine Destroy Ozone.
From www.chemtube3d.com
A Level Radical Reactions CFCs and the Ozone Layer How Does Chlorine And Bromine Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. chlorine, bromine, or iodine, is added to the stratosphere, the local ozone loss rates will generally increase, resulting in more. They also show that the reactive chemical chlorine. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic”. How Does Chlorine And Bromine Destroy Ozone.
From pdfprof.com
What are the chlorine and bromine reactions that destroy PDF How Does Chlorine And Bromine Destroy Ozone — when chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules. the tropospheric equivalent chlorine (ec) abundance is the starting point for calculating the stratospheric halogen. numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated. research studies in the laboratory show that chlorine (cl). How Does Chlorine And Bromine Destroy Ozone.
From www.theozonehole.org
The Ozone Hole How Does Chlorine And Bromine Destroy Ozone bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. the tropospheric equivalent chlorine (ec) abundance is the starting point for calculating the stratospheric halogen. — these changes in atmospheric chemical composition. How Does Chlorine And Bromine Destroy Ozone.
From slideplayer.com
بسم الّله الرّحمن الرّحیم ppt download How Does Chlorine And Bromine Destroy Ozone — when chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. They also show that the reactive chemical chlorine. — chlorine acts as a catalyst in turning ozone into oxygen in a. How Does Chlorine And Bromine Destroy Ozone.
From pwonlyias.com
Ozone Layer Depletion Causes, Effects And Solution How Does Chlorine And Bromine Destroy Ozone bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction. — when chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules. — learn how chlorine and bromine radicals catalytically destroy ozone molecules in the stratosphere, leading to. the tropospheric equivalent chlorine (ec) abundance. How Does Chlorine And Bromine Destroy Ozone.
From www.kindpng.com
Chemical Process Of Ozone Depletion, HD Png Download kindpng How Does Chlorine And Bromine Destroy Ozone chlorine, bromine, or iodine, is added to the stratosphere, the local ozone loss rates will generally increase, resulting in more. bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction. They also show that the reactive chemical chlorine. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made. How Does Chlorine And Bromine Destroy Ozone.
From ar.inspiredpencil.com
The Effects Of Ozone Layer How Does Chlorine And Bromine Destroy Ozone learn how chlorine atoms catalytically destroy ozone molecules in the stratosphere, and how cfcs and other gases are involved in ozone depletion. research studies in the laboratory show that chlorine (cl) reacts very rapidly with ozone. — chlorine acts as a catalyst in turning ozone into oxygen in a reaction that wasn't fully understood until 1973. . How Does Chlorine And Bromine Destroy Ozone.
From www.acsh.org
Know Your Ozone It's Good, Bad and BacteriaBlasting American How Does Chlorine And Bromine Destroy Ozone bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. each chlorine atom would react immediately with an ozone molecule, setting off a chain reaction that would destroy thousands of. reactive gases. How Does Chlorine And Bromine Destroy Ozone.
From www.slideserve.com
PPT Q9 What are the chlorine and bromine reactions that destroy How Does Chlorine And Bromine Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. each chlorine atom would react immediately with an ozone molecule, setting off a chain reaction that would destroy thousands of. numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated. — chlorine acts as. How Does Chlorine And Bromine Destroy Ozone.
From www.researchgate.net
Diagram showing the effect of polar stratospheric clouds on ozone loss How Does Chlorine And Bromine Destroy Ozone chlorine, bromine, or iodine, is added to the stratosphere, the local ozone loss rates will generally increase, resulting in more. learn how chlorine atoms catalytically destroy ozone molecules in the stratosphere, and how cfcs and other gases are involved in ozone depletion. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of. How Does Chlorine And Bromine Destroy Ozone.
From www.atmo.arizona.edu
ATMO336 Spring 2020 How Does Chlorine And Bromine Destroy Ozone learn how chlorine atoms catalytically destroy ozone molecules in the stratosphere, and how cfcs and other gases are involved in ozone depletion. the tropospheric equivalent chlorine (ec) abundance is the starting point for calculating the stratospheric halogen. bromine destroys ozone 50 times more efficiently than chlorine, the most infamous player in ozone destruction. reactive gases containing. How Does Chlorine And Bromine Destroy Ozone.
From www.slideserve.com
PPT Ozone Depletion PowerPoint Presentation, free download ID1994903 How Does Chlorine And Bromine Destroy Ozone — chlorine acts as a catalyst in turning ozone into oxygen in a reaction that wasn't fully understood until 1973. each chlorine atom would react immediately with an ozone molecule, setting off a chain reaction that would destroy thousands of. research studies in the laboratory show that chlorine (cl) reacts very rapidly with ozone. They also show. How Does Chlorine And Bromine Destroy Ozone.
From www.britannica.com
Ozone Depletion Saving Earth Encyclopedia Britannica How Does Chlorine And Bromine Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. — chlorine acts as a catalyst in turning ozone into oxygen in a reaction that wasn't fully understood until 1973. — learn how chlorine and bromine radicals catalytically destroy ozone molecules in the stratosphere, leading to. bromine destroys. How Does Chlorine And Bromine Destroy Ozone.