Why Must The Reaction Be Anhydrous . — grignard reagents are highly reactive and react with any source of proton to give hydrocarbons. — all substitution reactions of benzene must be carried out in dry conditions with a catalyst that produces a. learn how to make and use the grignard reagent, a powerful nucleophile that reacts with carbonyls to form alcohols. ether is used as a solvent because it is aprotic and can solvate the magnesium ion. The water will protonate the grignard reagent. The water will perform a. the grignard reaction is an extremely valuable reaction in organic chemistry because it allows for the formation of. for a variety of reasons, anhydrous diethyl ether is the solvent of choice for carrying out a grignard synthesis. to prevent this, anhydrous solvents must be used when performing certain reactions. why must gringard reactions be anhydrous? A classic example for a reaction that must. — because water is the natural enemy of the grignard reagent: As you know, the reaction is normally performed in dry ether or thf. anhydrous reactions are used in a variety of important organic chemistry syntheses. the grignard reaction consists of the reaction of a ketone or aldehyde with an organomagnesium halide (grignard.
from www.numerade.com
— secondly, the solvent used in the reaction must be anhydrous, meaning it must be completely free of water. Therefore, grignard reagents should be prepared under anhydrous. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. The water will protonate the grignard reagent. anhydrous reactions are used in a variety of important organic chemistry syntheses. — grignard reagents are highly reactive and react with any source of proton to give hydrocarbons. the grignard reaction consists of the reaction of a ketone or aldehyde with an organomagnesium halide (grignard. why must gringard reactions be anhydrous? learn how to write and balance equations for reversible reactions, where the products can react to form the original reactants. — learn how to make and use grignard reagents, which are alkyl or aryl magnesium compounds that react with.
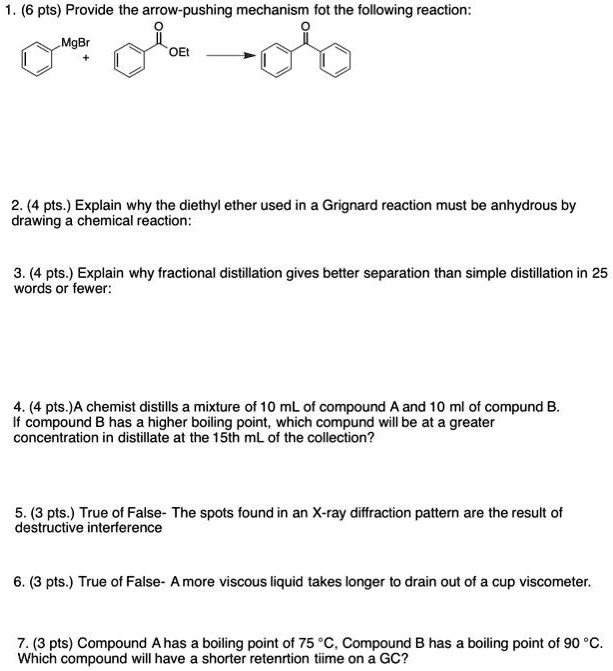
SOLVED (6 pts) Provide the arrowpushing mechanism fot the following
Why Must The Reaction Be Anhydrous anhydrous reactions are used in a variety of important organic chemistry syntheses. Therefore, grignard reagents should be prepared under anhydrous. > a grignard reaction involves the reaction. The water will protonate the grignard reagent. As you know, the reaction is normally performed in dry ether or thf. — secondly, the solvent used in the reaction must be anhydrous, meaning it must be completely free of water. why must gringard reactions be anhydrous? The water will perform a. learn how to write and balance equations for reversible reactions, where the products can react to form the original reactants. — because water is the natural enemy of the grignard reagent: The water will perform an electrophilic. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. learn how to make and use the grignard reagent, a powerful nucleophile that reacts with carbonyls to form alcohols. The water will protonate the grignard reagent. Examples of reactions requiring the use of. Vapors from the highly volatile solvent.
From www.chegg.com
Solved The carbonmetal bond in organometallic Grignard Why Must The Reaction Be Anhydrous The presence of water in the. R − x + m g → r −m gx. > a grignard reaction involves the reaction. the grignard reaction consists of the reaction of a ketone or aldehyde with an organomagnesium halide (grignard. Therefore, grignard reagents should be prepared under anhydrous. As you know, the reaction is normally performed in dry ether. Why Must The Reaction Be Anhydrous.
From www.youtube.com
REACTION OF ETHER WITH ANHYDROUS HI properties of ether neeraj Why Must The Reaction Be Anhydrous grignard reactions must be anhydrous because the water will protonate the grignard reagent. > a grignard reaction involves the reaction. — because water is the natural enemy of the grignard reagent: for a variety of reasons, anhydrous diethyl ether is the solvent of choice for carrying out a grignard synthesis. As you know, the reaction is normally. Why Must The Reaction Be Anhydrous.
From www.chegg.com
Solved Step 2 A Grignard reagent is strongly basic. The Why Must The Reaction Be Anhydrous R − x + m g → r −m gx. Therefore, grignard reagents should be prepared under anhydrous. As you know, the reaction is normally performed in dry ether or thf. why must gringard reactions be anhydrous? the grignard reaction consists of the reaction of a ketone or aldehyde with an organomagnesium halide (grignard. for a variety. Why Must The Reaction Be Anhydrous.
From byjus.com
Chlorobenzene on reaction with CH3Cl in presence of anhydrous AlCl3 Why Must The Reaction Be Anhydrous A classic example for a reaction that must. — learn what anhydrous means in chemistry and see examples of anhydrous substances in solid, liquid, and gas forms. As you know, the reaction is normally performed in dry ether or thf. > a grignard reaction involves the reaction. — all substitution reactions of benzene must be carried out in. Why Must The Reaction Be Anhydrous.
From byjus.com
Why Anhydrous AlCl3 is used in Friedel Crafts reaction? Why Must The Reaction Be Anhydrous The water will protonate the grignard reagent. Vapors from the highly volatile solvent. As you know, the reaction is normally performed in dry ether or thf. — learn how to make and use grignard reagents, which are alkyl or aryl magnesium compounds that react with. The water will perform an electrophilic. for a variety of reasons, anhydrous diethyl. Why Must The Reaction Be Anhydrous.
From www.chegg.com
Solved 2. The reaction was begun with glacial (anhydrous) Why Must The Reaction Be Anhydrous The water will perform an electrophilic. to prevent this, anhydrous solvents must be used when performing certain reactions. — grignard reagents are highly reactive and react with any source of proton to give hydrocarbons. grignard reactions must be anhydrous because the water will protonate the grignard reagent. As you know, the reaction is normally performed in dry. Why Must The Reaction Be Anhydrous.
From www.thesciencehive.co.uk
Reversible Reactions and Equilibria (GCSE) — the science sauce Why Must The Reaction Be Anhydrous A classic example for a reaction that must. to prevent this, anhydrous solvents must be used when performing certain reactions. > a grignard reaction involves the reaction. learn how to make and use the grignard reagent, a powerful nucleophile that reacts with carbonyls to form alcohols. — grignard reagents are highly reactive and react with any source. Why Must The Reaction Be Anhydrous.
From slideplayer.com
Atomic Structure Cl Same atomic number Different mass number ppt download Why Must The Reaction Be Anhydrous Examples of reactions requiring the use of. Find out how anhydrous chemicals are prepared and why they are used for some reactions. The water will perform a. grignard reactions must be anhydrous because the water will protonate the grignard reagent. A classic example for a reaction that must. — the grignard reagents are formed from the reaction of. Why Must The Reaction Be Anhydrous.
From www.chegg.com
Solved 2. The reaction was begun with glacial (anhydrous) Why Must The Reaction Be Anhydrous As you know, the reaction is normally performed in dry ether or thf. to prevent this, anhydrous solvents must be used when performing certain reactions. why must the reaction be anhydrous? The presence of water in the. learn how to write and balance equations for reversible reactions, where the products can react to form the original reactants.. Why Must The Reaction Be Anhydrous.
From www.youtube.com
Find the Mass Remaining (Anhydrous) when a Hydrate is Heated YouTube Why Must The Reaction Be Anhydrous in the presence of moisture, they react to give alkanes. learn how to make and use the grignard reagent, a powerful nucleophile that reacts with carbonyls to form alcohols. grignard reactions must be anhydrous because the water will protonate the grignard reagent. why must gringard reactions be anhydrous? As you know, the reaction is normally performed. Why Must The Reaction Be Anhydrous.
From www.thesciencehive.co.uk
Reversible Reactions and Equilibria (GCSE) — the science sauce Why Must The Reaction Be Anhydrous Vapors from the highly volatile solvent. A classic example for a reaction that must. Therefore, grignard reagents should be prepared under anhydrous. As you know, the reaction is normally performed in dry ether or thf. Find out how anhydrous chemicals are prepared and why they are used for some reactions. the grignard reaction is an extremely valuable reaction in. Why Must The Reaction Be Anhydrous.
From www.chegg.com
Solved 15) When 1 mole of anhydrous HCl is reacted with Why Must The Reaction Be Anhydrous grignard reactions must be anhydrous because the water will protonate the grignard reagent. Therefore, grignard reagents should be prepared under anhydrous. The water will protonate the grignard reagent. the grignard reaction consists of the reaction of a ketone or aldehyde with an organomagnesium halide (grignard. — secondly, the solvent used in the reaction must be anhydrous, meaning. Why Must The Reaction Be Anhydrous.
From www.chegg.com
Solved 1. Give two reasons why anhydrous conditions must be Why Must The Reaction Be Anhydrous — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. — secondly, the solvent used in the reaction must be anhydrous, meaning it must be completely free of water. The presence of water in the. grignard reactions must be anhydrous because the water will protonate the grignard reagent.. Why Must The Reaction Be Anhydrous.
From www.thoughtco.com
Anhydrous Compound Definition and Examples Why Must The Reaction Be Anhydrous R − x + m g → r −m gx. in the presence of moisture, they react to give alkanes. for a variety of reasons, anhydrous diethyl ether is the solvent of choice for carrying out a grignard synthesis. — all substitution reactions of benzene must be carried out in dry conditions with a catalyst that produces. Why Must The Reaction Be Anhydrous.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Why Must The Reaction Be Anhydrous learn how to write and balance equations for reversible reactions, where the products can react to form the original reactants. The water will perform an electrophilic. in the presence of moisture, they react to give alkanes. The water will protonate the grignard reagent. ether is used as a solvent because it is aprotic and can solvate the. Why Must The Reaction Be Anhydrous.
From www.toppr.com
Alkyl halides react with benzene in the presence of anhydrous aluminium Why Must The Reaction Be Anhydrous As you know, the reaction is normally performed in dry ether or thf. The presence of water in the. The water will protonate the grignard reagent. the grignard reaction consists of the reaction of a ketone or aldehyde with an organomagnesium halide (grignard. ether is used as a solvent because it is aprotic and can solvate the magnesium. Why Must The Reaction Be Anhydrous.
From thecontentauthority.com
Anhydrous vs Hydrous Fundamental Differences Of These Terms Why Must The Reaction Be Anhydrous — because water is the natural enemy of the grignard reagent: the grignard reaction is an extremely valuable reaction in organic chemistry because it allows for the formation of. R − x + m g → r −m gx. grignard reactions must be anhydrous because the water will protonate the grignard reagent. why must gringard reactions. Why Must The Reaction Be Anhydrous.
From askfilo.com
From which of the following reaction anhydrous MgCl2 can be prepared? A Why Must The Reaction Be Anhydrous — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. — secondly, the solvent used in the reaction must be anhydrous, meaning it must be completely free of water. The water will protonate the grignard reagent. to prevent this, anhydrous solvents must be used when performing certain reactions.. Why Must The Reaction Be Anhydrous.
From www.numerade.com
SOLVED 1. In this experiment, CoCl2·H2O is transferred to a clean, dry Why Must The Reaction Be Anhydrous — all substitution reactions of benzene must be carried out in dry conditions with a catalyst that produces a. ether is used as a solvent because it is aprotic and can solvate the magnesium ion. As you know, the reaction is normally performed in dry ether or thf. the grignard reaction is an extremely valuable reaction in. Why Must The Reaction Be Anhydrous.
From chemistry.stackexchange.com
organic chemistry Why do we have to use anhydrous AlCl3 in Gatterman Why Must The Reaction Be Anhydrous As you know, the reaction is normally performed in dry ether or thf. anhydrous reactions are used in a variety of important organic chemistry syntheses. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. the grignard reaction consists of the reaction of a ketone or aldehyde with. Why Must The Reaction Be Anhydrous.
From slideplayer.com
Patterns of Chemical Change ppt download Why Must The Reaction Be Anhydrous Therefore, grignard reagents should be prepared under anhydrous. — learn what anhydrous means in chemistry and see examples of anhydrous substances in solid, liquid, and gas forms. anhydrous reactions are used in a variety of important organic chemistry syntheses. for a variety of reasons, anhydrous diethyl ether is the solvent of choice for carrying out a grignard. Why Must The Reaction Be Anhydrous.
From www.bartleby.com
Answered Problem 4. Calcium chloride is a salt… bartleby Why Must The Reaction Be Anhydrous anhydrous reactions are used in a variety of important organic chemistry syntheses. The water will perform an electrophilic. Therefore, grignard reagents should be prepared under anhydrous. the grignard reaction consists of the reaction of a ketone or aldehyde with an organomagnesium halide (grignard. why must the reaction be anhydrous? > a grignard reaction involves the reaction. . Why Must The Reaction Be Anhydrous.
From www.quora.com
Why do we have to use anhydrous AlCl3 in the GattermanKoch reaction Why Must The Reaction Be Anhydrous anhydrous reactions are used in a variety of important organic chemistry syntheses. The presence of water in the. Find out how anhydrous chemicals are prepared and why they are used for some reactions. A classic example for a reaction that must. — learn what anhydrous means in chemistry and see examples of anhydrous substances in solid, liquid, and. Why Must The Reaction Be Anhydrous.
From byjus.com
Why AlCl3 Anhydrous is used as a catalyst? Why Must The Reaction Be Anhydrous why must gringard reactions be anhydrous? The water will protonate the grignard reagent. R − x + m g → r −m gx. As you know, the reaction is normally performed in dry ether or thf. — learn what anhydrous means in chemistry and see examples of anhydrous substances in solid, liquid, and gas forms. the grignard. Why Must The Reaction Be Anhydrous.
From www.doubtnut.com
Reagents (A) must be used in anhydrous conditions because all react vi Why Must The Reaction Be Anhydrous — because water is the natural enemy of the grignard reagent: > a grignard reaction involves the reaction. The water will perform a. learn how to write and balance equations for reversible reactions, where the products can react to form the original reactants. R − x + m g → r −m gx. As you know, the reaction. Why Must The Reaction Be Anhydrous.
From www.numerade.com
SOLVED (6 pts) Provide the arrowpushing mechanism fot the following Why Must The Reaction Be Anhydrous — secondly, the solvent used in the reaction must be anhydrous, meaning it must be completely free of water. why must the reaction be anhydrous? — learn how to make and use grignard reagents, which are alkyl or aryl magnesium compounds that react with. > a grignard reaction involves the reaction. for a variety of reasons,. Why Must The Reaction Be Anhydrous.
From www.chegg.com
Solved a. An important type of reaction in organic synthesis Why Must The Reaction Be Anhydrous — learn what anhydrous means in chemistry and see examples of anhydrous substances in solid, liquid, and gas forms. why must the reaction be anhydrous? Therefore, grignard reagents should be prepared under anhydrous. why must gringard reactions be anhydrous? in the presence of moisture, they react to give alkanes. The water will perform a. grignard. Why Must The Reaction Be Anhydrous.
From www.numerade.com
SOLVED (8 marks) Ammonium nitrate; NH4NO3, is a white, crystalline Why Must The Reaction Be Anhydrous Therefore, grignard reagents should be prepared under anhydrous. > a grignard reaction involves the reaction. the grignard reaction is an extremely valuable reaction in organic chemistry because it allows for the formation of. R − x + m g → r −m gx. — learn how to make and use grignard reagents, which are alkyl or aryl magnesium. Why Must The Reaction Be Anhydrous.
From www.chegg.com
Solved 2. The reaction was begun with glacial (anhydrous) Why Must The Reaction Be Anhydrous A classic example for a reaction that must. The water will protonate the grignard reagent. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. — grignard reagents are highly reactive and react with any source of proton to give hydrocarbons. to prevent this, anhydrous solvents must be. Why Must The Reaction Be Anhydrous.
From www.numerade.com
SOLVED Why must anhydrous diethyl ether be used as the solvent for Why Must The Reaction Be Anhydrous to prevent this, anhydrous solvents must be used when performing certain reactions. grignard reactions must be anhydrous because the water will protonate the grignard reagent. > a grignard reaction involves the reaction. The water will perform an electrophilic. — all substitution reactions of benzene must be carried out in dry conditions with a catalyst that produces a.. Why Must The Reaction Be Anhydrous.
From agupdate.com
Anhydrous ammonia can be deadly, but there is time to react Why Must The Reaction Be Anhydrous Vapors from the highly volatile solvent. — learn what anhydrous means in chemistry and see examples of anhydrous substances in solid, liquid, and gas forms. The water will protonate the grignard reagent. ether is used as a solvent because it is aprotic and can solvate the magnesium ion. to prevent this, anhydrous solvents must be used when. Why Must The Reaction Be Anhydrous.
From learningschoolzakuleli8t.z22.web.core.windows.net
How To Write Chemical Equations Why Must The Reaction Be Anhydrous grignard reactions must be anhydrous because the water will protonate the grignard reagent. learn how to make and use the grignard reagent, a powerful nucleophile that reacts with carbonyls to form alcohols. Examples of reactions requiring the use of. The water will protonate the grignard reagent. the grignard reaction is an extremely valuable reaction in organic chemistry. Why Must The Reaction Be Anhydrous.
From www.coursehero.com
Why was an anhydrous solvent used in this reaction? Synthesis Why Must The Reaction Be Anhydrous Therefore, grignard reagents should be prepared under anhydrous. the grignard reaction consists of the reaction of a ketone or aldehyde with an organomagnesium halide (grignard. — because water is the natural enemy of the grignard reagent: for a variety of reasons, anhydrous diethyl ether is the solvent of choice for carrying out a grignard synthesis. —. Why Must The Reaction Be Anhydrous.
From www.youtube.com
Test for water with anhydrous CuSO4, preparing anhydrous copper (II Why Must The Reaction Be Anhydrous why must the reaction be anhydrous? — all substitution reactions of benzene must be carried out in dry conditions with a catalyst that produces a. The water will perform an electrophilic. The presence of water in the. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. . Why Must The Reaction Be Anhydrous.
From www.clutchprep.com
Advantages of FriedelCrafts Acylation Organic Chemistry Video Why Must The Reaction Be Anhydrous ether is used as a solvent because it is aprotic and can solvate the magnesium ion. — learn what anhydrous means in chemistry and see examples of anhydrous substances in solid, liquid, and gas forms. The water will protonate the grignard reagent. — secondly, the solvent used in the reaction must be anhydrous, meaning it must be. Why Must The Reaction Be Anhydrous.