Ground Term Of Nitrogen . Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. To assign transitions in an electronic spectrum, we should know the ground state term, as well as all other terms and their relative energies for a. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Find out how to calculate the quantum numbers l, ml, ms and s for. The ground state electron configuration of ground state gaseous neutral. Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. The lowest energy term is that which has the greatest spin multiplicity. I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. Now, the rule's for determining the ground state term are:
from present5.com
I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. Find out how to calculate the quantum numbers l, ml, ms and s for. Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. Now, the rule's for determining the ground state term are: Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. To assign transitions in an electronic spectrum, we should know the ground state term, as well as all other terms and their relative energies for a. Nitrogen atoms have 7 electrons and the shell structure is 2.5. The lowest energy term is that which has the greatest spin multiplicity. The ground state electron configuration of ground state gaseous neutral.
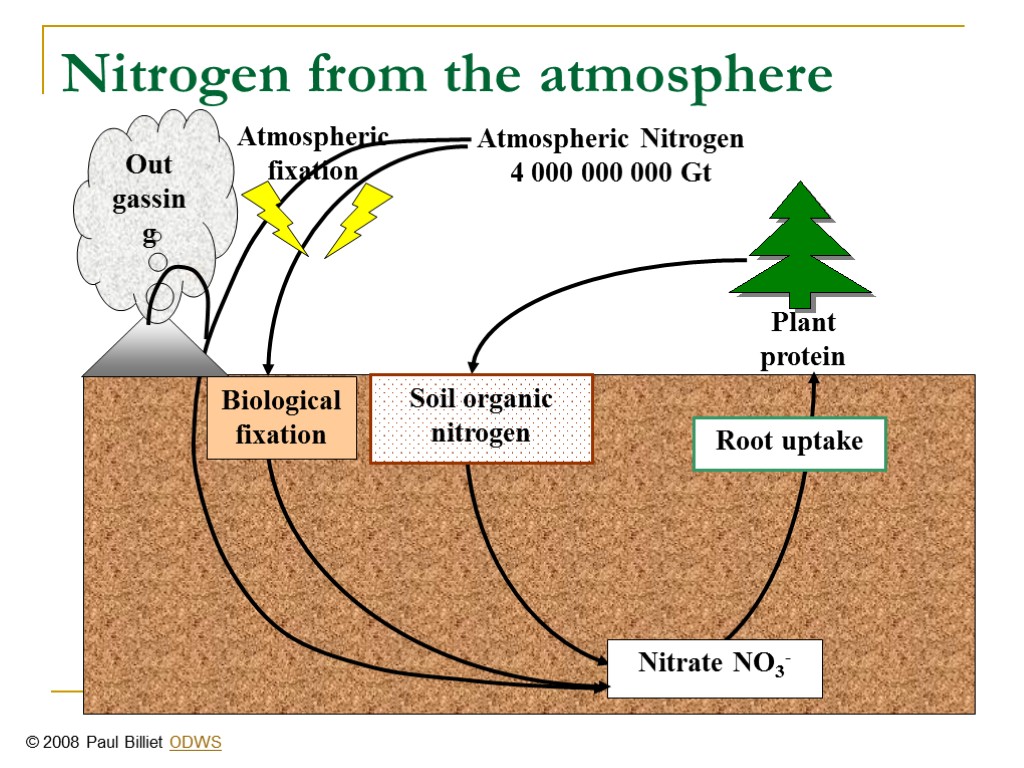
THE NITROGEN CYCLENitrates are essential for plant growth
Ground Term Of Nitrogen The ground state electron configuration of ground state gaseous neutral. Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. The lowest energy term is that which has the greatest spin multiplicity. I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. The ground state electron configuration of ground state gaseous neutral. To assign transitions in an electronic spectrum, we should know the ground state term, as well as all other terms and their relative energies for a. Now, the rule's for determining the ground state term are: Nitrogen atoms have 7 electrons and the shell structure is 2.5. Find out how to calculate the quantum numbers l, ml, ms and s for. Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells.
From www.earth.com
Why is the Nitrogen Cycle So Important? Ground Term Of Nitrogen The lowest energy term is that which has the greatest spin multiplicity. To assign transitions in an electronic spectrum, we should know the ground state term, as well as all other terms and their relative energies for a. I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. Find out how to. Ground Term Of Nitrogen.
From blog-crop-news.extension.umn.edu
How the nitrogen cycle affects fall N availability Ground Term Of Nitrogen Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. The ground state electron configuration of ground state gaseous neutral. To assign transitions in an electronic spectrum, we should know the ground. Ground Term Of Nitrogen.
From www.sciencelearn.org.nz
The nitrogen cycle — Science Learning Hub Ground Term Of Nitrogen Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. To assign transitions in. Ground Term Of Nitrogen.
From www.lessonpaths.com
Image Map Of The Nitrogen Cycle What Happens In The Soil? LessonPaths Ground Term Of Nitrogen Find out how to calculate the quantum numbers l, ml, ms and s for. Now, the rule's for determining the ground state term are: The ground state electron configuration of ground state gaseous neutral. Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. Nitrogen atoms have 7 electrons and the shell. Ground Term Of Nitrogen.
From ecologyconnections.weebly.com
Nitrogen Cycle Ecology Connections Ground Term Of Nitrogen Find out how to calculate the quantum numbers l, ml, ms and s for. Now, the rule's for determining the ground state term are: The lowest energy term is that which has the greatest spin multiplicity. Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. Look up for the possible term. Ground Term Of Nitrogen.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID2037968 Ground Term Of Nitrogen Now, the rule's for determining the ground state term are: Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. To assign transitions in an electronic spectrum, we should know the ground state term, as well as all other terms and their relative energies for a. Nitrogen atoms have 7 electrons and. Ground Term Of Nitrogen.
From www.researchgate.net
6 Pathways of nitrogen in marine surface sediments. Arrows black Ground Term Of Nitrogen Now, the rule's for determining the ground state term are: Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. Find out how to calculate the quantum numbers l, ml, ms and s for. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Learn how to use term. Ground Term Of Nitrogen.
From www.researchgate.net
44 Nitrogen ground levels, first excited states (n=3) and the Ground Term Of Nitrogen I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. Find out how to calculate the quantum numbers l, ml, ms and s for. The lowest energy term is that which has the greatest spin multiplicity. Learn how to use term symbols to describe an atom's electronic state based on its unfilled. Ground Term Of Nitrogen.
From www.yourdictionary.com
Nitrogen Cycle Examples YourDictionary Ground Term Of Nitrogen Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. Find out how to calculate the quantum numbers l, ml, ms and s for. I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. The ground state electron configuration of ground state gaseous neutral.. Ground Term Of Nitrogen.
From www.science-sparks.com
What is the Nitrogen Cycle? Science for Kids Ground Term Of Nitrogen Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. The ground state electron configuration of ground state gaseous neutral. I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. Nitrogen atoms have 7 electrons and the shell structure is 2.5. The. Ground Term Of Nitrogen.
From www.sciencefacts.net
Nitrogen Cycle Definition, Steps, Importance with Diagram Ground Term Of Nitrogen The lowest energy term is that which has the greatest spin multiplicity. Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Now, the rule's for determining the ground state term are: Find out how to calculate the quantum numbers l,. Ground Term Of Nitrogen.
From www.onlinebiologynotes.com
Nitrogen cycle Steps of Nitrogen cycle Online Biology Notes Ground Term Of Nitrogen Find out how to calculate the quantum numbers l, ml, ms and s for. Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. Nitrogen atoms have 7 electrons and the shell structure is. Ground Term Of Nitrogen.
From present5.com
THE NITROGEN CYCLENitrates are essential for plant growth Ground Term Of Nitrogen Now, the rule's for determining the ground state term are: To assign transitions in an electronic spectrum, we should know the ground state term, as well as all other terms and their relative energies for a. I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. Learn how to use term symbols. Ground Term Of Nitrogen.
From potatoesnz.co.nz
NitrogeninfographicFINAL Potatoes New Zealand Ground Term Of Nitrogen Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. Find out how to calculate the quantum numbers l, ml, ms and s for. Now, the rule's for determining the ground state term are: Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition. Ground Term Of Nitrogen.
From www.numerade.com
SOLVED Determine the term symbols for nitrogen and indicate the ground Ground Term Of Nitrogen To assign transitions in an electronic spectrum, we should know the ground state term, as well as all other terms and their relative energies for a. Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. Learn how to use term symbols to describe an atom's electronic state based on. Ground Term Of Nitrogen.
From www.slideserve.com
PPT The Nitrogen Cycle PowerPoint Presentation, free download ID Ground Term Of Nitrogen The ground state electron configuration of ground state gaseous neutral. Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. To assign transitions in an electronic spectrum, we should know the ground. Ground Term Of Nitrogen.
From serc.carleton.edu
The Nitrogen Cycle and Human Management of Soils Ground Term Of Nitrogen Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. The ground state electron configuration of ground state gaseous neutral. Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. I'm trying to figure out what terms are possible for nitrogen with. Ground Term Of Nitrogen.
From www.researchgate.net
Analytical flow of total nitrogen and nitrogen in soil Ground Term Of Nitrogen I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. The ground state electron configuration of ground state gaseous neutral. The. Ground Term Of Nitrogen.
From www.toppr.com
Ground state configuration of nitrogen atom can be Ground Term Of Nitrogen Now, the rule's for determining the ground state term are: Nitrogen atoms have 7 electrons and the shell structure is 2.5. To assign transitions in an electronic spectrum, we should know the ground state term, as well as all other terms and their relative energies for a. Find out how to calculate the quantum numbers l, ml, ms and s. Ground Term Of Nitrogen.
From www.researchgate.net
Nitrogen cycle in terms of major biological pathways for Download Ground Term Of Nitrogen Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. Find out how to calculate the quantum numbers l, ml, ms and s for. The lowest energy term is that which has the greatest spin multiplicity. Now, the rule's for determining the ground state term are: The ground state electron configuration of. Ground Term Of Nitrogen.
From www.algaebarn.com
Understanding the Nitrogen Cycle Beginners Education AlgaeBarn Ground Term Of Nitrogen The ground state electron configuration of ground state gaseous neutral. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. The lowest energy term is that which has the greatest spin multiplicity. Now, the rule's for determining the ground. Ground Term Of Nitrogen.
From rachel-yersblogdalton.blogspot.com
Explain Different Steps of Nitrogen Cycle Ground Term Of Nitrogen The lowest energy term is that which has the greatest spin multiplicity. The ground state electron configuration of ground state gaseous neutral. Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. Find out how to calculate the quantum numbers l, ml, ms and s for. Nitrogen atoms have 7. Ground Term Of Nitrogen.
From www.pinterest.com
Nitrogen Fixation Easy Science Nitrogen fixation, Biology facts Ground Term Of Nitrogen I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. The ground state electron configuration of ground state gaseous neutral. To assign transitions in an electronic spectrum, we should know the ground state term, as well as all other terms and their relative energies for a. Now, the rule's for determining the. Ground Term Of Nitrogen.
From www.teachoo.com
Nitrogen Cycle Diagram with Steps Explained Teachoo Concepts Ground Term Of Nitrogen Find out how to calculate the quantum numbers l, ml, ms and s for. Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. I'm trying to figure out what terms are possible for nitrogen with the electron configuration $\ce{[he] 2s^2 2p^3}$. The ground state electron configuration of ground state gaseous neutral.. Ground Term Of Nitrogen.
From mavink.com
Diagram Of Nitrogen Cycle Ground Term Of Nitrogen Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. The ground state electron configuration of ground state gaseous neutral. The lowest energy term is that which has the greatest spin multiplicity. To assign transitions in an electronic spectrum, we should know the ground state term, as well as all. Ground Term Of Nitrogen.
From www.artofit.org
Nitrogen cycle Artofit Ground Term Of Nitrogen The lowest energy term is that which has the greatest spin multiplicity. Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Find out how to calculate the quantum numbers l, ml, ms and s for. To assign transitions. Ground Term Of Nitrogen.
From water.unl.edu
Nitrogen Dynamics UNL Water Ground Term Of Nitrogen Nitrogen atoms have 7 electrons and the shell structure is 2.5. Now, the rule's for determining the ground state term are: Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. Find out how to calculate the quantum numbers l, ml, ms and s for. To assign transitions in an electronic spectrum,. Ground Term Of Nitrogen.
From www.youtube.com
The Nitrogen Cycle! YouTube Ground Term Of Nitrogen Now, the rule's for determining the ground state term are: Find out how to calculate the quantum numbers l, ml, ms and s for. The ground state electron configuration of ground state gaseous neutral. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Look up for the possible term symbols for the d 2 configuration, and determine how. Ground Term Of Nitrogen.
From socratic.org
How does nitrogen cycle through the biosphere? Socratic Ground Term Of Nitrogen Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. To assign transitions in an electronic spectrum, we should know the ground state term, as well as all other terms and their relative energies for a. Find out how to calculate the quantum numbers l, ml, ms and s for.. Ground Term Of Nitrogen.
From qcebiologyrevision.com
Nitrogen Cycle QCE Biology Revision Ground Term Of Nitrogen The lowest energy term is that which has the greatest spin multiplicity. Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. Nitrogen atoms have 7 electrons and the shell structure is 2.5. To assign transitions in an electronic spectrum, we should know the ground state term, as well as all other. Ground Term Of Nitrogen.
From www.teachmint.com
Rules To Find Ground Term Symbol Chemistry Notes Teachmint Ground Term Of Nitrogen The ground state electron configuration of ground state gaseous neutral. Nitrogen atoms have 7 electrons and the shell structure is 2.5. Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. Find out how to calculate the quantum numbers l, ml, ms and s for. I'm trying to figure out. Ground Term Of Nitrogen.
From byjus.com
Nitrogen Cycle Explained Definition, Stages and Importance Ground Term Of Nitrogen Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. To assign transitions in an electronic spectrum, we should know the ground state term, as well as all other terms and their relative energies for a. Find out how to calculate the quantum numbers l, ml, ms and s for. I'm trying. Ground Term Of Nitrogen.
From brandonkss.github.io
Ground State Electron Configuration Chart Ground Term Of Nitrogen Find out how to calculate the quantum numbers l, ml, ms and s for. Now, the rule's for determining the ground state term are: Nitrogen atoms have 7 electrons and the shell structure is 2.5. Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. I'm trying to figure out. Ground Term Of Nitrogen.
From www.researchgate.net
The nitrogen cycle. The main pool of N in the soil is in the form of Ground Term Of Nitrogen Now, the rule's for determining the ground state term are: The ground state electron configuration of ground state gaseous neutral. The lowest energy term is that which has the greatest spin multiplicity. Find out how to calculate the quantum numbers l, ml, ms and s for. Learn how to use term symbols to describe an atom's electronic state based on. Ground Term Of Nitrogen.
From agriculturalinformation4u.com
Nitrogen Nutrient in Soil Management and its Role in Agriculture crop Ground Term Of Nitrogen Now, the rule's for determining the ground state term are: Look up for the possible term symbols for the d 2 configuration, and determine how many fundamentals (transition from the. Learn how to use term symbols to describe an atom's electronic state based on its unfilled sub shells. The ground state electron configuration of ground state gaseous neutral. Nitrogen atoms. Ground Term Of Nitrogen.