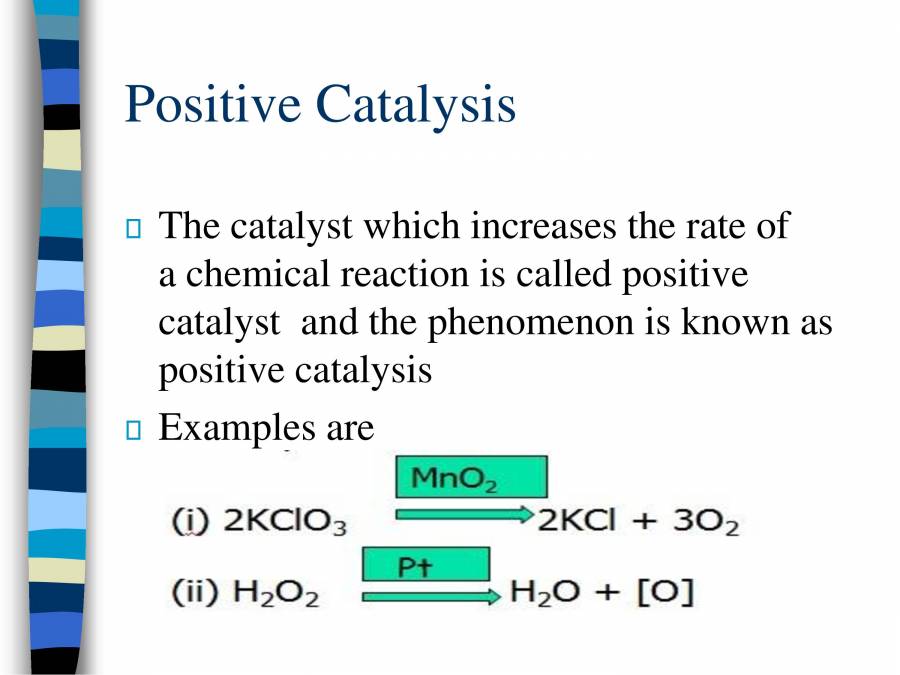
Positive Catalyst And Inhibitor Difference . Describe the similarities and differences between the three principal classes of catalysts. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. On the other hand, a negative catalyst is unable to decrease the. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific reactions. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur. Define physisorption and chemisorption, and explain the role of the. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation.
from www.learnpick.in
Describe the similarities and differences between the three principal classes of catalysts. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur. A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. Define physisorption and chemisorption, and explain the role of the. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. On the other hand, a negative catalyst is unable to decrease the. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific reactions.
Catalysis PowerPoint Slides LearnPick India
Positive Catalyst And Inhibitor Difference A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific reactions. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. Define physisorption and chemisorption, and explain the role of the. On the other hand, a negative catalyst is unable to decrease the. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Describe the similarities and differences between the three principal classes of catalysts.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Positive Catalyst And Inhibitor Difference A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific reactions. On the other hand, a negative catalyst is unable to decrease the. Usually when someone refers to a catalyst, they mean a positive. Positive Catalyst And Inhibitor Difference.
From slideplayer.com
Introduction to Chemistry Chapter ppt download Positive Catalyst And Inhibitor Difference A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific reactions.. Positive Catalyst And Inhibitor Difference.
From slideplayer.com
Chemical Reactions Types, Energy, and Rates ppt download Positive Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. Define physisorption and chemisorption, and explain the role of the. A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur. Usually when. Positive Catalyst And Inhibitor Difference.
From www.differencebetween.com
Difference Between Catalyst and Inhibitor Compare the Difference Positive Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. On the other hand, a negative catalyst is unable to decrease the. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. A catalyst speeds up chemical reactions without. Positive Catalyst And Inhibitor Difference.
From slideplayer.com
CHEMICAL KINITICS Kononova T.O.. ppt download Positive Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. Define physisorption and chemisorption, and explain the role of the. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. A catalyst speeds up chemical reactions without being consumed,. Positive Catalyst And Inhibitor Difference.
From www.youtube.com
Catalyst and Inhibitors YouTube Positive Catalyst And Inhibitor Difference Define physisorption and chemisorption, and explain the role of the. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific reactions. A catalyst speeds up chemical reactions. Positive Catalyst And Inhibitor Difference.
From ablogwithadifference.com
Catalyst and Inhibitor The best 5 difference Positive Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. On the other hand, a negative catalyst is unable to decrease the. Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific reactions. A catalyst is a substance that increases the. Positive Catalyst And Inhibitor Difference.
From philschatz.com
Energy, Matter, and Enzymes · Microbiology Positive Catalyst And Inhibitor Difference A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. Describe the similarities and differences between the three principal classes of catalysts. On the other hand, a negative catalyst is unable to decrease the. Usually when someone refers to a catalyst, they mean a positive catalyst, which is. Positive Catalyst And Inhibitor Difference.
From www.youtube.com
Catalyst Promoter and Catalyst Inhibitor Chemistry YouTube Positive Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. On the other hand, a negative catalyst is unable to decrease the. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. A catalyst is a substance that increases. Positive Catalyst And Inhibitor Difference.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Positive Catalyst And Inhibitor Difference A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific reactions. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. A catalyst speeds. Positive Catalyst And Inhibitor Difference.
From in.pinterest.com
Positive vs Negative Catalyst Tabular Form Positive Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. Define physisorption and chemisorption, and explain the role of the. Negative catalysts can. Positive Catalyst And Inhibitor Difference.
From differencebtw.com
Catalyst vs. Inhibitor Know the Difference Positive Catalyst And Inhibitor Difference A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur. Describe the similarities and differences between the three principal classes of catalysts. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. A positive catalyst. Positive Catalyst And Inhibitor Difference.
From pediaa.com
What is the Difference Between Enzyme Activator and Enzyme Inhibitor Positive Catalyst And Inhibitor Difference This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Describe the similarities and differences between the three principal classes of catalysts. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur. Usually. Positive Catalyst And Inhibitor Difference.
From thecontentauthority.com
Catalyst vs Inhibitor Which Should You Use In Writing? Positive Catalyst And Inhibitor Difference Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific reactions. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of. Positive Catalyst And Inhibitor Difference.
From www.learnpick.in
Catalysis PowerPoint Slides LearnPick India Positive Catalyst And Inhibitor Difference A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a. Positive Catalyst And Inhibitor Difference.
From unacademy.com
Positive Catalysts Positive Catalyst And Inhibitor Difference Define physisorption and chemisorption, and explain the role of the. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur. A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. On the other hand, a negative catalyst is unable to decrease the. A catalyst. Positive Catalyst And Inhibitor Difference.
From www.youtube.com
B.7.2 Compare catalysts and biological catalysts (enzymes Positive Catalyst And Inhibitor Difference A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. Define physisorption and chemisorption, and explain the role of the. Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific reactions. Describe the similarities and differences between the three principal classes of catalysts. On. Positive Catalyst And Inhibitor Difference.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Positive Catalyst And Inhibitor Difference On the other hand, a negative catalyst is unable to decrease the. Describe the similarities and differences between the three principal classes of catalysts. Define physisorption and chemisorption, and explain the role of the. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. This page looks at. Positive Catalyst And Inhibitor Difference.
From www.slideserve.com
PPT General Chemistry PowerPoint Presentation, free download ID1047081 Positive Catalyst And Inhibitor Difference A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur. Describe the similarities and differences between the three principal classes of catalysts. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction. Positive Catalyst And Inhibitor Difference.
From byjus.com
Which of the following statement is incorrect about positive and Positive Catalyst And Inhibitor Difference This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur.. Positive Catalyst And Inhibitor Difference.
From slideplayer.com
ppt download Positive Catalyst And Inhibitor Difference A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur. Define physisorption and chemisorption, and explain the role of the. A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down. Positive Catalyst And Inhibitor Difference.
From www.slideshare.net
Catalysis Positive Catalyst And Inhibitor Difference Define physisorption and chemisorption, and explain the role of the. On the other hand, a negative catalyst is unable to decrease the. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of. Positive Catalyst And Inhibitor Difference.
From www.slideserve.com
PPT Catalysts and inhibitors, But Mostly Catalysts PowerPoint Positive Catalyst And Inhibitor Difference A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. Define physisorption and chemisorption, and explain the role of the. On the other hand, a negative catalyst is unable to decrease the. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Negative. Positive Catalyst And Inhibitor Difference.
From scienceinfo.com
Catalyst and Catalysis Types, Examples, Differences Positive Catalyst And Inhibitor Difference A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. Describe the similarities and differences between the three principal classes of catalysts. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. A catalyst speeds up chemical reactions. Positive Catalyst And Inhibitor Difference.
From www.slideserve.com
PPT & Equilibrium PowerPoint Presentation, free download Positive Catalyst And Inhibitor Difference Define physisorption and chemisorption, and explain the role of the. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur. A positive catalyst. Positive Catalyst And Inhibitor Difference.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation ID2683753 Positive Catalyst And Inhibitor Difference A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. On the other hand, a negative catalyst is unable to decrease the. Describe the similarities and differences between the three principal classes of catalysts. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required for the reaction to occur.. Positive Catalyst And Inhibitor Difference.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Positive Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. On the. Positive Catalyst And Inhibitor Difference.
From www.askdifference.com
Catalyst vs. Inhibitor — What’s the Difference? Positive Catalyst And Inhibitor Difference A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. Define physisorption and chemisorption, and explain the role of the. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Describe the similarities and differences between the three principal classes of catalysts. On. Positive Catalyst And Inhibitor Difference.
From slideplayer.com
Catalysts Rates of Reactions. ppt download Positive Catalyst And Inhibitor Difference Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy required. Positive Catalyst And Inhibitor Difference.
From www.slideserve.com
PPT Observing and describing chemical reactions PowerPoint Positive Catalyst And Inhibitor Difference On the other hand, a negative catalyst is unable to decrease the. Describe the similarities and differences between the three principal classes of catalysts. Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific reactions. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of. Positive Catalyst And Inhibitor Difference.
From www.animalia-life.club
Enzyme Graph Transition State Positive Catalyst And Inhibitor Difference This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. Define physisorption and chemisorption, and explain the. Positive Catalyst And Inhibitor Difference.
From www.3ditalian.com
Difference Between Catalyst and Inhibitor Compare the Difference Positive Catalyst And Inhibitor Difference On the other hand, a negative catalyst is unable to decrease the. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. Describe the similarities and differences between. Positive Catalyst And Inhibitor Difference.
From www.slideserve.com
PPT Chapter 21 Section 4 PowerPoint Presentation, free download ID Positive Catalyst And Inhibitor Difference Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific reactions. Define physisorption and chemisorption, and explain the role of the. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. On the other hand, a negative. Positive Catalyst And Inhibitor Difference.
From www.differencebetween.com
Difference Between Catalyst and Inhibitor Compare the Difference Positive Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. On the other hand, a negative catalyst is unable to decrease the. Usually when someone refers to a catalyst, they mean a positive catalyst,. Positive Catalyst And Inhibitor Difference.
From www.youtube.com
Catalysts and Inhibitors YouTube Positive Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. Define physisorption and chemisorption, and explain the role of the. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Negative catalysts can selectively inhibit certain reactions, while positive catalysts can selectively enhance specific. Positive Catalyst And Inhibitor Difference.