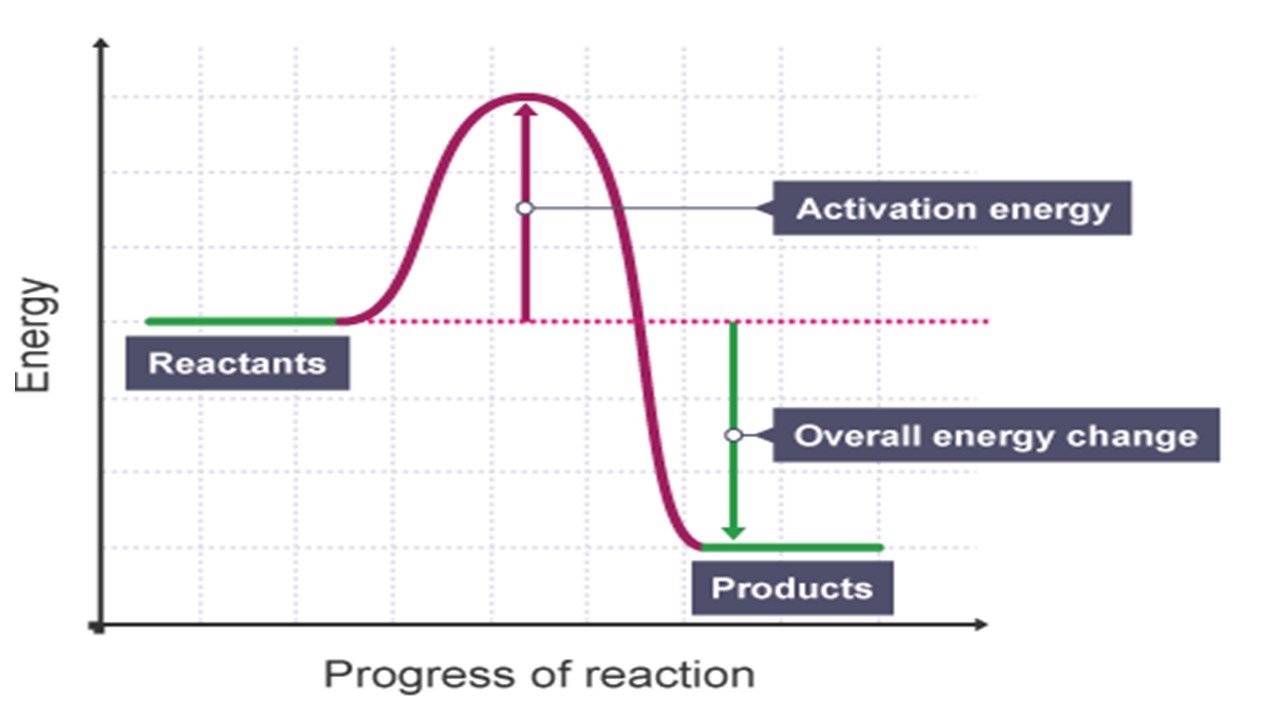
Endothermic Activation Energy . Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Activation energy is the minimum amount of energy transferred to the reactants that allows a chemical reaction to start. Learn how to calculate and graph. Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. And a reaction profile shows the. Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. They cause the reaction mixture and its surroundings to become colder. Find out how temperature and catalysts affect activation energy and rate of reaction. Activation energy is the minimum energy needed to start a chemical reaction. A reaction with a high activation energy is slow and requires external energy to proceed. Learn how to define and identify endothermic and exothermic reactions based on heat transfer and energy diagrams.
from telegra.ph
Learn how to define and identify endothermic and exothermic reactions based on heat transfer and energy diagrams. They cause the reaction mixture and its surroundings to become colder. Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Find out how temperature and catalysts affect activation energy and rate of reaction. A reaction with a high activation energy is slow and requires external energy to proceed. Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. Activation energy is the minimum energy needed to start a chemical reaction. Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams.
Endothermic energy profile diagram activation Telegraph
Endothermic Activation Energy Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. Learn how to calculate and graph. Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. Activation energy is the minimum energy needed to start a chemical reaction. Activation energy is the minimum amount of energy transferred to the reactants that allows a chemical reaction to start. A reaction with a high activation energy is slow and requires external energy to proceed. Find out how temperature and catalysts affect activation energy and rate of reaction. Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. Learn how to define and identify endothermic and exothermic reactions based on heat transfer and energy diagrams. They cause the reaction mixture and its surroundings to become colder. And a reaction profile shows the.
From www.istockphoto.com
Vector Graphs Of Endothermic And Exothermic Reactions Activation Energy Endothermic Activation Energy They cause the reaction mixture and its surroundings to become colder. Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. Activation energy. Endothermic Activation Energy.
From www.youtube.com
ACTIVATION ENERGY WITH GRAPH FOR EXOTHERMIC AND ENDOTHERMIC REACTION L1 Endothermic Activation Energy Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Learn how to define and identify endothermic and exothermic reactions based on heat transfer and energy diagrams. Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. And a reaction profile shows the. Endothermic reactions are those that take. Endothermic Activation Energy.
From www.expii.com
Endothermic and Exothermic Reactions — Overview & Comparison Expii Endothermic Activation Energy And a reaction profile shows the. Find out how temperature and catalysts affect activation energy and rate of reaction. Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. Learn how to define and identify endothermic and exothermic. Endothermic Activation Energy.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Activation Energy They cause the reaction mixture and its surroundings to become colder. Learn how to define and identify endothermic and exothermic reactions based on heat transfer and energy diagrams. Learn how to calculate and graph. Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. Find out how temperature and catalysts affect activation energy and rate. Endothermic Activation Energy.
From www.slideserve.com
PPT Exothermic & Endothermic Reactions Energy Diagrams PowerPoint Endothermic Activation Energy And a reaction profile shows the. Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. They cause the reaction mixture and its surroundings to become colder. Learn how to calculate and graph. Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Learn what endothermic reactions are, how. Endothermic Activation Energy.
From quizlet.com
Year 10 Chemistry Exothermic and Endothermic Reactions Diagram Endothermic Activation Energy Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. Learn how to calculate and graph. Activation energy is the minimum amount of. Endothermic Activation Energy.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Activation Energy Learn how to define and identify endothermic and exothermic reactions based on heat transfer and energy diagrams. Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Activation energy is the minimum amount of energy transferred to the reactants. Endothermic Activation Energy.
From elecdiags.com
The Significance and Use of Endothermic Reaction Profile Diagrams in Endothermic Activation Energy Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. Activation energy is the minimum energy needed to start a chemical reaction. Activation energy is the minimum amount of energy transferred to the reactants that allows a chemical reaction to start. They cause the reaction mixture and its surroundings to become colder. Learn what endothermic. Endothermic Activation Energy.
From www.dreamstime.com
Activation Energy Endothermic Reaction 2d Illustration Stock Endothermic Activation Energy Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. A reaction with a high activation energy is slow and requires external energy to proceed. They cause the reaction mixture and its surroundings to become colder. And a reaction profile shows the. Learn how to draw reaction profiles and understand activation energy for. Endothermic Activation Energy.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Activation Energy Activation energy is the minimum amount of energy transferred to the reactants that allows a chemical reaction to start. Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. A reaction with a high activation energy is slow and requires external energy to proceed. Activation energy is the minimum energy needed to start a chemical. Endothermic Activation Energy.
From stock.adobe.com
activation energy endothermic reaction diagram vector de Stock Adobe Endothermic Activation Energy Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. And a reaction profile shows the. They cause the reaction mixture and its surroundings to become colder. A reaction with a high activation energy is slow and. Endothermic Activation Energy.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID5592422 Endothermic Activation Energy A reaction with a high activation energy is slow and requires external energy to proceed. Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. Learn how to calculate and graph. They cause the reaction mixture and its surroundings to become colder. Activation energy is the minimum energy barrier that molecules must overcome to react. Endothermic Activation Energy.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Activation Energy Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. A reaction with a high activation energy is slow and requires external energy to proceed. Activation energy is the minimum energy needed to start a chemical reaction. Learn. Endothermic Activation Energy.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Activation Energy A reaction with a high activation energy is slow and requires external energy to proceed. Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Activation energy is the minimum energy needed to start a chemical reaction. They cause the reaction mixture and its surroundings to become colder. Find out how temperature and catalysts. Endothermic Activation Energy.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Activation Energy Activation energy is the minimum energy barrier that molecules must overcome to react and form products. And a reaction profile shows the. Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. Find out how temperature and catalysts affect activation energy and rate of reaction. They cause the reaction mixture and its surroundings to become. Endothermic Activation Energy.
From studylib.net
Lesson 7 Reaction profiles Endothermic Activation Energy Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Learn how to calculate and graph. And a reaction profile shows the. Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. Find out how temperature and catalysts affect activation energy and rate of reaction. They cause. Endothermic Activation Energy.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Activation Energy Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. Learn how to define and identify endothermic and exothermic reactions based on heat transfer and energy diagrams. They cause the reaction mixture and its surroundings to become colder. Learn. Endothermic Activation Energy.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Activation Energy Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. And a reaction profile shows the. Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. Learn how to define and identify endothermic and. Endothermic Activation Energy.
From www.toppr.com
Consider the endothermic reaction, X Y , with the activation energies Endothermic Activation Energy Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. Activation energy is the minimum amount of energy transferred to the reactants that allows a chemical reaction to start. Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. Activation energy is the minimum energy needed to start. Endothermic Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Endothermic Activation Energy Activation energy is the minimum amount of energy transferred to the reactants that allows a chemical reaction to start. They cause the reaction mixture and its surroundings to become colder. Activation energy is the minimum energy needed to start a chemical reaction. Learn how to define and identify endothermic and exothermic reactions based on heat transfer and energy diagrams. Learn. Endothermic Activation Energy.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Endothermic Activation Energy Learn how to calculate and graph. Find out how temperature and catalysts affect activation energy and rate of reaction. And a reaction profile shows the. Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. Activation energy is the minimum amount of energy transferred to the reactants that allows a chemical reaction to start. Activation. Endothermic Activation Energy.
From schematicmandorlas.z14.web.core.windows.net
Energy Diagram For An Endothermic Reaction Endothermic Activation Energy They cause the reaction mixture and its surroundings to become colder. Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Learn what endothermic. Endothermic Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Endothermic Activation Energy Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. A reaction with a high activation energy is slow and requires external energy to proceed. Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. They cause the reaction mixture and its surroundings to become colder. Activation energy is the. Endothermic Activation Energy.
From printablelibzeloso.z21.web.core.windows.net
Endothermic Vs Exothermic Reactions Examples Endothermic Activation Energy A reaction with a high activation energy is slow and requires external energy to proceed. Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. And a reaction profile shows the. Learn how to calculate and graph. Learn how. Endothermic Activation Energy.
From www.doubtnut.com
For an endothermic reaction energy of activation is E(a) and enthlpy o Endothermic Activation Energy Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. A reaction with a high activation energy is slow and requires external energy to proceed. Learn how to calculate and graph. And a reaction profile shows the. Activation energy is the minimum amount of energy transferred to the reactants that allows a chemical. Endothermic Activation Energy.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation ID239015 Endothermic Activation Energy Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. Learn how to define and identify endothermic and exothermic reactions based on heat transfer and energy diagrams. Learn how to calculate and graph. A reaction with a high activation energy is slow and requires external energy to proceed. Endothermic reactions are those that take in. Endothermic Activation Energy.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Endothermic Activation Energy Activation energy is the minimum energy needed to start a chemical reaction. Activation energy is the minimum amount of energy transferred to the reactants that allows a chemical reaction to start. Learn how to define and identify endothermic and exothermic reactions based on heat transfer and energy diagrams. Find out how temperature and catalysts affect activation energy and rate of. Endothermic Activation Energy.
From manualdbbedecked.z13.web.core.windows.net
Energy Diagram For Endothermic Reaction Endothermic Activation Energy Activation energy is the minimum energy barrier that molecules must overcome to react and form products. Activation energy is the minimum energy needed to start a chemical reaction. A reaction with a high activation energy is slow and requires external energy to proceed. Learn how to calculate and graph. Find out how temperature and catalysts affect activation energy and rate. Endothermic Activation Energy.
From telegra.ph
Endothermic energy profile diagram activation Telegraph Endothermic Activation Energy Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. Activation energy is the minimum energy barrier that molecules must overcome to react and form products. A reaction with a high activation energy is slow and requires external energy to proceed. Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic. Endothermic Activation Energy.
From www.slideserve.com
PPT Energy Changes PowerPoint Presentation, free download ID3470966 Endothermic Activation Energy They cause the reaction mixture and its surroundings to become colder. Activation energy is the minimum energy needed to start a chemical reaction. A reaction with a high activation energy is slow and requires external energy to proceed. Learn how to calculate and graph. And a reaction profile shows the. Learn how to draw reaction profiles and understand activation energy. Endothermic Activation Energy.
From spmchemistry.blog.onlinetuition.com.my
Collision Theory SPM Chemistry Endothermic Activation Energy They cause the reaction mixture and its surroundings to become colder. And a reaction profile shows the. Activation energy is the minimum amount of energy transferred to the reactants that allows a chemical reaction to start. Learn how to calculate and graph. Find out how temperature and catalysts affect activation energy and rate of reaction. Learn how to define and. Endothermic Activation Energy.
From schematicdbelfriede.z13.web.core.windows.net
Energy Diagram For Endothermic Reaction Endothermic Activation Energy They cause the reaction mixture and its surroundings to become colder. Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. A reaction with a high activation energy is slow and requires external energy to proceed. Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. Learn how. Endothermic Activation Energy.
From telegra.ph
Endothermic energy profile diagram activation Telegraph Endothermic Activation Energy Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. A reaction with a high activation energy is slow and requires external energy to proceed. Activation energy is the minimum energy needed to start a chemical reaction. And a reaction profile shows the. Learn how to define and identify endothermic and exothermic reactions based on. Endothermic Activation Energy.
From byjus.com
24. What is the Minimum activation energy required for endothermic and Endothermic Activation Energy Learn how to identify exothermic and endothermic reactions using energy level diagrams and reaction profiles. Learn how to calculate and graph. They cause the reaction mixture and its surroundings to become colder. Activation energy is the minimum energy needed to start a chemical reaction. Endothermic reactions are those that take in energy from the surroundings, usually as heat energy. And. Endothermic Activation Energy.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Activation Energy Learn how to draw reaction profiles and understand activation energy for exothermic and endothermic reactions. They cause the reaction mixture and its surroundings to become colder. Learn what endothermic reactions are, how to identify them, and how to represent them with energy diagrams. Learn how to define and identify endothermic and exothermic reactions based on heat transfer and energy diagrams.. Endothermic Activation Energy.