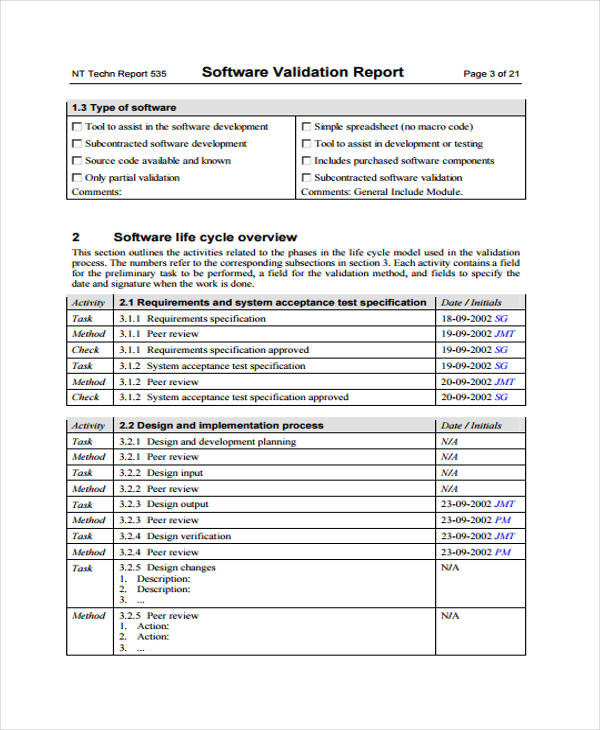
Analytical Instrument Validation . Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. Right instrument and system for the job: Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. Right analytical method for the job: If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use.
from www.template.net
All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. Right analytical method for the job: Right instrument and system for the job: Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories.
10+ Validation Report Templates Free Sample, Example Format Download
Analytical Instrument Validation Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. Right instrument and system for the job: The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. Right analytical method for the job: Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories.
From www.americanpharmaceuticalreview.com
Analytical Method Validation for Quality Assurance and Process Analytical Instrument Validation The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. Right analytical method. Analytical Instrument Validation.
From www.drugfuture.com
ANALYTICAL INSTRUMENT QUALIFICATION Analytical Instrument Validation The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. If you are working. Analytical Instrument Validation.
From www.researchgate.net
Template of a validation certificate. Download Scientific Diagram Analytical Instrument Validation All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. Right instrument and. Analytical Instrument Validation.
From pharmabeginers.com
Analytical Method Transfer (USP 1224) Guideline Pharma Beginners Analytical Instrument Validation Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. Right instrument. Analytical Instrument Validation.
From www.semanticscholar.org
Analytical Instrument Qualification Standardization on the 4Q Model Analytical Instrument Validation Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. All analytical instruments and systems need t o. Analytical Instrument Validation.
From www.semanticscholar.org
Figure 1 from An Integrated Risk Assessment for Analytical Instruments Analytical Instrument Validation The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Two of the. Analytical Instrument Validation.
From ciqa.net
How to create a Validation Master Plan in 5 steps. Templates & more Analytical Instrument Validation If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. Right analytical method for the job: Right instrument and system for the job: All analytical instruments and systems need t o be subject. Analytical Instrument Validation.
From instrumentationforum.com
Instrumentation Calibration Forms Instrumentation Instrumentation Forum Analytical Instrument Validation Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. If you are. Analytical Instrument Validation.
From www.azom.com
Analytical Method Validation in Thermal Analysis Analytical Instrument Validation Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. Right analytical method for the job: All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach. Analytical Instrument Validation.
From www.semanticscholar.org
AN OVERVIEW OF ANALYTICAL INSTRUMENT QUALIFICATION WITH REFERENCE OF Analytical Instrument Validation Right analytical method for the job: Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. Right instrument and system for the job: There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. Two of the primary sources of guidance for the verification of analytical instruments. Analytical Instrument Validation.
From www.researchgate.net
(PDF) A Review HPLC Method Development and Validation Analytical Instrument Validation All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. Right analytical method for the job: Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. There are many. Analytical Instrument Validation.
From www.spectroscopyeurope.com
Instrument qualification a possible quality by designbased approach Analytical Instrument Validation Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Right analytical method. Analytical Instrument Validation.
From www.slideserve.com
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download Analytical Instrument Validation Right analytical method for the job: The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Right instrument and system for the job: There are many ways of demonstrating that an instrument is. Analytical Instrument Validation.
From www.qlaboratories.com
Analytical Chemistry Laboratory Services Q Laboratories Analytical Instrument Validation Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. All analytical instruments and. Analytical Instrument Validation.
From www.researchgate.net
(PDF) An integrated and harmonized approach to analytical instrument Analytical Instrument Validation The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Right analytical method for. Analytical Instrument Validation.
From www.slideshare.net
Analytical Instrument Qualification and System Validation Analytical Instrument Validation Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. Right analytical method for the job: Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. There are. Analytical Instrument Validation.
From www.scribd.com
Validation Criteria Checklist Verification And Validation Analytical Instrument Validation There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. Right instrument and system for the job: If. Analytical Instrument Validation.
From worldcomplianceseminars.com
Analytical Instrument Qualification and System Validation WCS Analytical Instrument Validation Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. There are many ways of demonstrating that an instrument. Analytical Instrument Validation.
From www.inpaspages.com
Calibration Status Verification Report format Analytical Instrument Validation Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. There are many ways of. Analytical Instrument Validation.
From www.template.net
Validation Report Templates 9+ Free Word, PDF Format Download Analytical Instrument Validation All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. Right instrument and system for the job: There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. If. Analytical Instrument Validation.
From www.biospectrumasia.com
How to Comply with the 2017 Version of USP? Analytical Instrument Validation There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. Right analytical method for the job: Analytical method validation is the collection of documented evidence that an analytical procedure is suitable. Analytical Instrument Validation.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Analytical Instrument Validation Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. Right analytical method for the job: There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. Analytical method validation is the collection of documented evidence that an analytical procedure is suitable. Analytical Instrument Validation.
From www.researchgate.net
(PDF) An Integrated Risk Assessment for Analytical Instruments and Analytical Instrument Validation All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. The eca analytical quality. Analytical Instrument Validation.
From snap-tech.com
Announces Virtual Seminar on Analytical Instrument Analytical Instrument Validation There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. All analytical instruments. Analytical Instrument Validation.
From www.researchgate.net
(PDF) Validation Instrument for Undergraduate Qualitative Research Analytical Instrument Validation Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. The eca. Analytical Instrument Validation.
From www.youtube.com
Improve validation accuracy YouTube Analytical Instrument Validation There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. The eca analytical quality control group (aqcg). Analytical Instrument Validation.
From dokumen.tips
(PDF) Analytical Instrument Qualification and System Validation Analytical Instrument Validation There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. Analytical validation/verification. Analytical Instrument Validation.
From gahess.com
Best Practices for Instrument Validation and Qualification (2022) Analytical Instrument Validation Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. Right instrument and system. Analytical Instrument Validation.
From www.chemwifi.com
Analytical Method validation for Pesticides, PCBs, PAHs Phthalates Analytical Instrument Validation There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. All analytical instruments and systems need t. Analytical Instrument Validation.
From www.semanticscholar.org
[PDF] An Integrated Risk Assessment for Analytical Instruments and Analytical Instrument Validation Right analytical method for the job: There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. All analytical instruments and systems need t o be subject to qualication as described in. Analytical Instrument Validation.
From www.researchgate.net
(PDF) AN OVERVIEW OF ANALYTICAL INSTRUMENT QUALIFICATION WITH REFERENCE Analytical Instrument Validation All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. There are many ways. Analytical Instrument Validation.
From www.scribd.com
Method Validation Report Template 1 Experiment Accuracy And Precision Analytical Instrument Validation Right instrument and system for the job: If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. Right analytical method for the job: Two of the primary sources of guidance for the verification of analytical instruments and computerized. Analytical Instrument Validation.
From www.semanticscholar.org
[PDF] Analytical Method Validation and Instrument Performance Analytical Instrument Validation The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. There are many ways of demonstrating that an instrument is qualified and under control, and these can include qualification, calibration,. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Two of the. Analytical Instrument Validation.
From mpl.loesungsfabrik.de
What is qualification? Analytical Instrument Validation Analytical method validation is the collection of documented evidence that an analytical procedure is suitable for its intended use. Two of the primary sources of guidance for the verification of analytical instruments and computerized systems in regulated laboratories. Analytical validation/verification laboratories are required to perform analytical validation or verification of each nonwaived. All analytical instruments and systems need t o. Analytical Instrument Validation.
From www.chromatographyonline.com
Understanding the Lifecycle Approach for Analytical Procedures Analytical Instrument Validation Right instrument and system for the job: All analytical instruments and systems need t o be subject to qualication as described in analytical instrument qualication. The eca analytical quality control group (aqcg) has developed a new guide for an integrated lifecycle approach to analytical. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api. Analytical Instrument Validation.