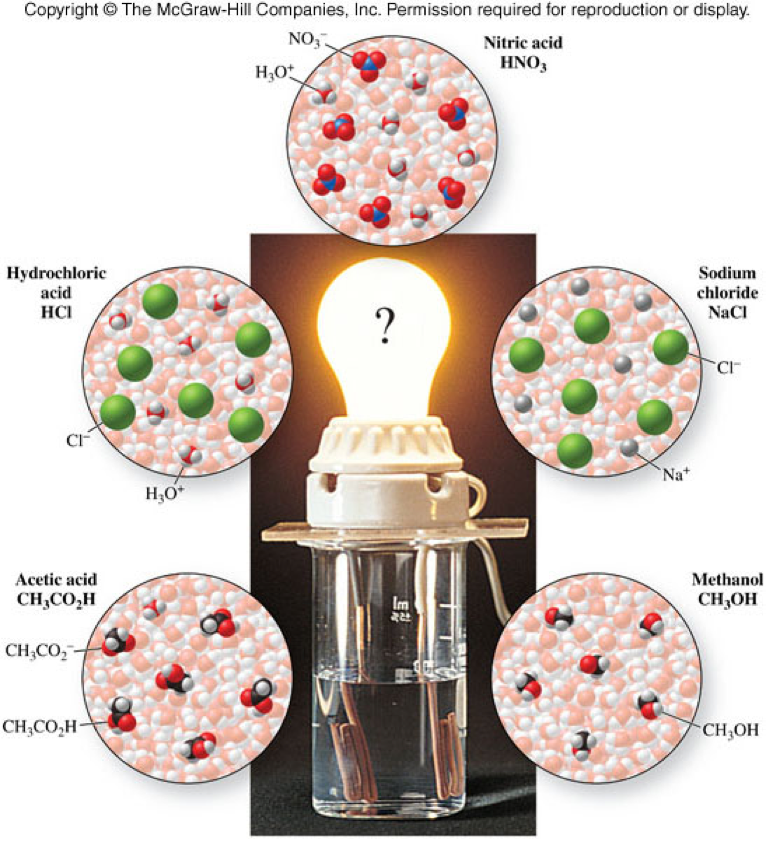
Can Ro Water Conduct Electricity . Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro water. Well actually, pure water is an excellent insulator and does not conduct electricity. What is conductivity in aqueous solutions? Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. The ions in the water conduct electricity because of their positive and negative charges. Measuring the conductivity of ro water helps determine how much salt is being rejected by the ro membrane. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. Water and electricity don't mix, right? Pure water is not a good conductor of electricity. At lower ph the ammonium. But how it actually conducts is the question. At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas). It can be simply explained by the following steps:
from ceritspm.blob.core.windows.net
What is conductivity in aqueous solutions? But how it actually conducts is the question. At lower ph the ammonium. It can be simply explained by the following steps: Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro water. Pure water is not a good conductor of electricity. Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. Water and electricity don't mix, right? The ions in the water conduct electricity because of their positive and negative charges. At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas).
Does Water Conduct Electricity at Misty Payne blog
Can Ro Water Conduct Electricity Well actually, pure water is an excellent insulator and does not conduct electricity. Water and electricity don't mix, right? Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. Measuring the conductivity of ro water helps determine how much salt is being rejected by the ro membrane. Pure water is not a good conductor of electricity. What is conductivity in aqueous solutions? The ions in the water conduct electricity because of their positive and negative charges. Well actually, pure water is an excellent insulator and does not conduct electricity. At lower ph the ammonium. But how it actually conducts is the question. It can be simply explained by the following steps: Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro water. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas).
From www.youtube.com
How can water conduct electricity? Simple Electric Circuit with Can Ro Water Conduct Electricity At lower ph the ammonium. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. Pure water is not a good conductor of electricity. Well actually, pure water is an excellent insulator and does not conduct electricity. Measuring the conductivity of ro water helps determine how much salt is being rejected by the. Can Ro Water Conduct Electricity.
From www.youtube.com
Conduction of Electricity Through Liquids (Electrolysis) Physics Can Ro Water Conduct Electricity Water and electricity don't mix, right? The ions in the water conduct electricity because of their positive and negative charges. Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro water. Pure water is not a good conductor of electricity. But how it actually conducts is the question. At higher ph the ammonia gas is prevalent,. Can Ro Water Conduct Electricity.
From loepxtfmp.blob.core.windows.net
Why Measure Electrical Conductivity In Water at Alma Mcghie blog Can Ro Water Conduct Electricity Pure water is not a good conductor of electricity. But how it actually conducts is the question. Well actually, pure water is an excellent insulator and does not conduct electricity. At lower ph the ammonium. It can be simply explained by the following steps: What is conductivity in aqueous solutions? Fortunately, ro treatment plants can monitor salt rejection by measuring. Can Ro Water Conduct Electricity.
From dxohnerux.blob.core.windows.net
Soap Electricity Conductivity at Debra Vaughn blog Can Ro Water Conduct Electricity At lower ph the ammonium. Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas). Fortunately, ro treatment plants can monitor salt rejection. Can Ro Water Conduct Electricity.
From www.teachoo.com
Difference between solids and liquids conducting electricity Teachoo Can Ro Water Conduct Electricity Well actually, pure water is an excellent insulator and does not conduct electricity. Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. At lower ph the ammonium. The ions in the water conduct electricity because of their positive and negative charges. Water and electricity don't mix,. Can Ro Water Conduct Electricity.
From exytxvzzy.blob.core.windows.net
How Electrolyte Solutions Conduct Electricity at Dewey Goodson blog Can Ro Water Conduct Electricity Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. What is conductivity in aqueous solutions? But how it actually conducts is the question. Water and electricity don't mix, right? Pure water is not a good conductor of electricity. Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro. Can Ro Water Conduct Electricity.
From msestudent.com
Why Do Metals Conduct Electricity? Materials Science & Engineering Can Ro Water Conduct Electricity What is conductivity in aqueous solutions? At lower ph the ammonium. Pure water is not a good conductor of electricity. At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas). Well actually, pure water is an excellent insulator and does not conduct electricity. Fortunately, ro treatment. Can Ro Water Conduct Electricity.
From www.youtube.com
5.2 Does water conduct electricity? YouTube Can Ro Water Conduct Electricity Well actually, pure water is an excellent insulator and does not conduct electricity. Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro water. Pure water is not a good conductor of electricity. Measuring the conductivity of ro water helps determine how much salt is being rejected by the ro membrane. Water can conduct electricity because. Can Ro Water Conduct Electricity.
From exotpzcki.blob.core.windows.net
Copper Wire Water Conduct Electricity at Rick Burson blog Can Ro Water Conduct Electricity Water and electricity don't mix, right? Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. At lower ph the ammonium. But how it actually conducts is the question. Measuring the conductivity of ro water helps determine how much salt is being rejected by the ro membrane.. Can Ro Water Conduct Electricity.
From www.youtube.com
Can water conduct Electricity? Pure vs Salt water Rohan YouTube Can Ro Water Conduct Electricity At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas). Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. Ordinary distilled water in equilibrium with carbon dioxide of the air has a. Can Ro Water Conduct Electricity.
From klahwrrnr.blob.core.windows.net
Which Water Can Conduct Electricity at Justin Jennings blog Can Ro Water Conduct Electricity But how it actually conducts is the question. At lower ph the ammonium. The ions in the water conduct electricity because of their positive and negative charges. Measuring the conductivity of ro water helps determine how much salt is being rejected by the ro membrane. Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro water.. Can Ro Water Conduct Electricity.
From ceritspm.blob.core.windows.net
Does Water Conduct Electricity at Misty Payne blog Can Ro Water Conduct Electricity Water and electricity don't mix, right? Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas). Well actually, pure water is an excellent. Can Ro Water Conduct Electricity.
From whatsinsight.org
Does Distilled Water Conduct Electricity? What's Insight Can Ro Water Conduct Electricity Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro water. It can be simply explained by the following steps: Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. Water and electricity don't mix, right? Measuring the conductivity of ro water helps determine how much salt is being. Can Ro Water Conduct Electricity.
From www.teachoo.com
Do liquids conduct electricity? Class 8 Science Notes Teachoo Can Ro Water Conduct Electricity Water and electricity don't mix, right? At lower ph the ammonium. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. Pure water is not a good conductor of electricity. Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro water. Well actually, pure water is an excellent insulator. Can Ro Water Conduct Electricity.
From www.youtube.com
electrical conductivity with salt water science experiment working Can Ro Water Conduct Electricity At lower ph the ammonium. Measuring the conductivity of ro water helps determine how much salt is being rejected by the ro membrane. Water and electricity don't mix, right? It can be simply explained by the following steps: Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. Pure water is not a. Can Ro Water Conduct Electricity.
From www.youtube.com
Does Water Really Conduct Electricity? YouTube Can Ro Water Conduct Electricity The ions in the water conduct electricity because of their positive and negative charges. It can be simply explained by the following steps: Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro water. What is conductivity in aqueous. Can Ro Water Conduct Electricity.
From www.youtube.com
Why Saltwater Conducts Electricity Science Experiment YouTube Can Ro Water Conduct Electricity At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas). Well actually, pure water is an excellent insulator and does not conduct electricity. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. But how it actually conducts is. Can Ro Water Conduct Electricity.
From www.youtube.com
Electrical conductivity with salt water YouTube Can Ro Water Conduct Electricity Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. Measuring the conductivity of ro water helps determine how much salt is being rejected by the ro membrane. It can be simply explained by the following steps: The ions in the water conduct electricity because of their. Can Ro Water Conduct Electricity.
From www.electricity-magnetism.org
¿Cómo conducen electricidad los electrolitos? Can Ro Water Conduct Electricity The ions in the water conduct electricity because of their positive and negative charges. But how it actually conducts is the question. At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas). Water can conduct electricity because of the ion concentration within the water, which comes. Can Ro Water Conduct Electricity.
From chemistrylabs-2.blogspot.com
Salt Water Electrical Conductivity Chemistry Labs Can Ro Water Conduct Electricity Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. At lower ph the ammonium. At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas). Water and electricity don't mix, right? It can be simply explained by the following. Can Ro Water Conduct Electricity.
From exolmohec.blob.core.windows.net
What Does Water Do To Electricity at Shelton Nicholson blog Can Ro Water Conduct Electricity At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas). At lower ph the ammonium. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro. Can Ro Water Conduct Electricity.
From www.youtube.com
Conductivity of Water What is Conductivity Conductivity of RO plant Can Ro Water Conduct Electricity What is conductivity in aqueous solutions? Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro water. Pure water is not a good conductor of electricity. At lower ph the ammonium. Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. Ordinary distilled. Can Ro Water Conduct Electricity.
From ceopyitw.blob.core.windows.net
What Compounds When Dissolved In Water Conduct Electricity at Jon Can Ro Water Conduct Electricity Pure water is not a good conductor of electricity. Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. What is conductivity in aqueous solutions? Measuring the conductivity of ro water helps determine how much salt is being rejected by the ro membrane. It can be simply. Can Ro Water Conduct Electricity.
From www.youtube.com
electrical conductivity with salt water working model school science Can Ro Water Conduct Electricity Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. What is conductivity in aqueous solutions? It can be simply explained by the following steps: Water and electricity don't mix,. Can Ro Water Conduct Electricity.
From www.rookieparenting.com
Electricity Science Projects Can Ro Water Conduct Electricity The ions in the water conduct electricity because of their positive and negative charges. Water and electricity don't mix, right? It can be simply explained by the following steps: But how it actually conducts is the question. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. Fortunately, ro treatment plants can monitor. Can Ro Water Conduct Electricity.
From www.studocu.com
1.PRAC Does Water Conduct Electricity Does Water Conduct Electricity Can Ro Water Conduct Electricity What is conductivity in aqueous solutions? Water and electricity don't mix, right? Measuring the conductivity of ro water helps determine how much salt is being rejected by the ro membrane. At lower ph the ammonium. At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas). Pure. Can Ro Water Conduct Electricity.
From atlas-scientific.com
What Is The Typical Water Conductivity Range? Atlas Scientific Can Ro Water Conduct Electricity It can be simply explained by the following steps: At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas). Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. But how it actually. Can Ro Water Conduct Electricity.
From www.youtube.com
Can Ro Water Be Used In Inverter Battery? YouTube Can Ro Water Conduct Electricity Well actually, pure water is an excellent insulator and does not conduct electricity. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. Water and electricity don't mix, right? At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas).. Can Ro Water Conduct Electricity.
From www.tessshebaylo.com
Water Undergoes Electrolysis Equation Tessshebaylo Can Ro Water Conduct Electricity Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. Measuring the conductivity of ro water helps determine how much salt is being rejected by the ro membrane. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. At lower. Can Ro Water Conduct Electricity.
From exoxmnlvb.blob.core.windows.net
Ro Water System Diagram at Cynthia Pack blog Can Ro Water Conduct Electricity Pure water is not a good conductor of electricity. At higher ph the ammonia gas is prevalent, and being a gas, will not be rejected by a ro (similar to carbon dioxide gas). But how it actually conducts is the question. Water and electricity don't mix, right? The ions in the water conduct electricity because of their positive and negative. Can Ro Water Conduct Electricity.
From learningmediablotted.z21.web.core.windows.net
Water Is Conductor Of Electricity Can Ro Water Conduct Electricity The ions in the water conduct electricity because of their positive and negative charges. At lower ph the ammonium. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. It can be simply explained by the following steps: Well actually, pure water is an excellent insulator and does not conduct electricity. Fortunately, ro. Can Ro Water Conduct Electricity.
From loepxtfmp.blob.core.windows.net
Why Measure Electrical Conductivity In Water at Alma Mcghie blog Can Ro Water Conduct Electricity Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro water. Pure water is not a good conductor of electricity. But how it actually conducts is the question. It can be simply explained by the following steps: Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic. Can Ro Water Conduct Electricity.
From www.youtube.com
Is pure distilled water conduct electricity ? ClassX, part9 YouTube Can Ro Water Conduct Electricity Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. It can be simply explained by the following steps: Measuring the conductivity of ro water helps determine how much salt is being rejected by the ro membrane. The ions in the water conduct electricity because of their positive and negative charges. Water can. Can Ro Water Conduct Electricity.
From techiescientist.com
Does Ice Conduct Electricity? Techiescientist Can Ro Water Conduct Electricity Measuring the conductivity of ro water helps determine how much salt is being rejected by the ro membrane. Pure water is not a good conductor of electricity. But how it actually conducts is the question. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10. Water and electricity don't mix, right? What is. Can Ro Water Conduct Electricity.
From www.rookieparenting.com
Does Water Conduct Electricity? Simple Experiment Can Ro Water Conduct Electricity Water can conduct electricity because of the ion concentration within the water, which comes from dissolved solids and inorganic materials like carbonate compounds,. Fortunately, ro treatment plants can monitor salt rejection by measuring the conductivity of ro water. Pure water is not a good conductor of electricity. At higher ph the ammonia gas is prevalent, and being a gas, will. Can Ro Water Conduct Electricity.