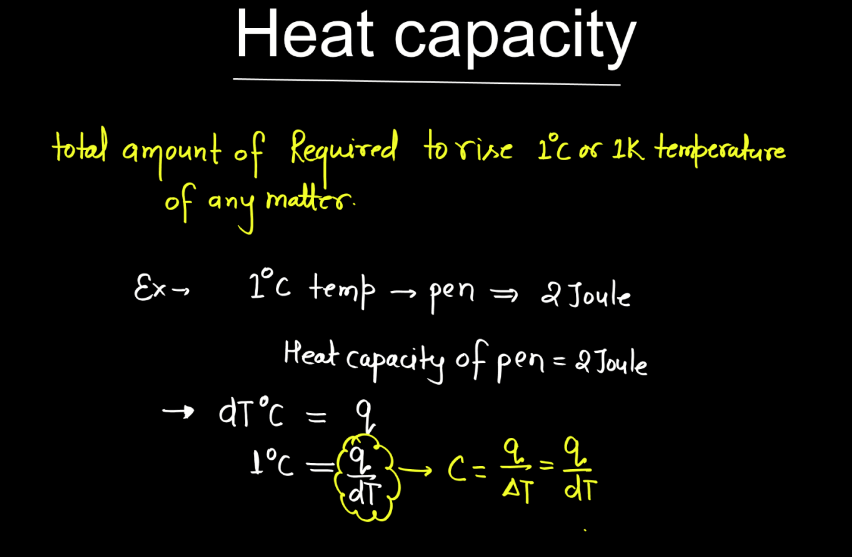
What Is Heat Capacity Gas . the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. define heat capacity of an ideal gas for a specific process; We can calculate it for an ideal gas. Heat capacity of an ideal. When we investigate the energy. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. here, we focus on the heat capacity with the volume held constant. Its si unit is j k. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\).
from www.careerpower.in
The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Heat capacity of an ideal. if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\). the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. here, we focus on the heat capacity with the volume held constant. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. Its si unit is j k. We can calculate it for an ideal gas. When we investigate the energy. define heat capacity of an ideal gas for a specific process;
What is Heat Capacity Definition, Equations, Examples and Types
What Is Heat Capacity Gas Its si unit is j k. Heat capacity of an ideal. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; here, we focus on the heat capacity with the volume held constant. if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\). When we investigate the energy. Its si unit is j k. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. define heat capacity of an ideal gas for a specific process; We can calculate it for an ideal gas.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Is Heat Capacity Gas When we investigate the energy. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; Its si unit is j k. define heat capacity of an ideal gas for a specific process; in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or. What Is Heat Capacity Gas.
From www.researchgate.net
6. Heat capacity of the flue gas depending on temperature and air What Is Heat Capacity Gas Its si unit is j k. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). here, we focus on the heat capacity with the volume held constant. the heat capacity (c) of a body of matter is the quantity of heat. What Is Heat Capacity Gas.
From jahamattbrown.blogspot.com
Specific Heat Capacity Definition Matt Brown What Is Heat Capacity Gas in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). Heat capacity of an ideal. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; if we heat or do work on any gas—real or ideal—the energy change. What Is Heat Capacity Gas.
From www.slideshare.net
Heat capacity heatofformation What Is Heat Capacity Gas the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. the specific heat (= specific heat capacity) at constant pressure. What Is Heat Capacity Gas.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity Gas if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\). Its si unit is j k. We can calculate it for an ideal gas. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). define heat capacity. What Is Heat Capacity Gas.
From www.youtube.com
Internal Energy of an Ideal Gas Molar Heat Capacity of Monatomic What Is Heat Capacity Gas We can calculate it for an ideal gas. define heat capacity of an ideal gas for a specific process; here, we focus on the heat capacity with the volume held constant. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). When. What Is Heat Capacity Gas.
From www.youtube.com
Example Using specific heat to calculate ideal gas internal energy What Is Heat Capacity Gas We can calculate it for an ideal gas. if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\). define heat capacity of an ideal gas for a specific process; Its si unit is j k. When we investigate the energy. here, we focus on the heat capacity with the volume held constant.. What Is Heat Capacity Gas.
From www.youtube.com
Specific heat capacity YouTube What Is Heat Capacity Gas in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. Its si unit is. What Is Heat Capacity Gas.
From www.youtube.com
20 Specific Heats of Ideal Gases&Liquids&Solids YouTube What Is Heat Capacity Gas define heat capacity of an ideal gas for a specific process; When we investigate the energy. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; here, we focus on the heat capacity with the volume held constant. the heat capacity (c) of a body of matter is the quantity of heat. What Is Heat Capacity Gas.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is Heat Capacity Gas the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases. What Is Heat Capacity Gas.
From www.tec-science.com
Specific heat capacity of gases (at constant volume or pressure) tec What Is Heat Capacity Gas define heat capacity of an ideal gas for a specific process; the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\). We can. What Is Heat Capacity Gas.
From www.tec-science.com
Specific heat capacity of selected substances tecscience What Is Heat Capacity Gas define heat capacity of an ideal gas for a specific process; When we investigate the energy. if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\). We can calculate it for an ideal gas. here, we focus on the heat capacity with the volume held constant. Its si unit is j k.. What Is Heat Capacity Gas.
From www.toppr.com
The pressure P of an ideal diatomic gas varies with its absolute What Is Heat Capacity Gas in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). We can calculate it for an ideal gas. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. if we heat or do work. What Is Heat Capacity Gas.
From www.grc.nasa.gov
Specific Heats What Is Heat Capacity Gas When we investigate the energy. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process;. What Is Heat Capacity Gas.
From www.youtube.com
3. 11P13.2 CV 2 Specific Heat Capacities of Gases and Mean Free Path What Is Heat Capacity Gas Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; When we investigate the energy. here, we focus on the heat capacity with the volume held constant. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt). What Is Heat Capacity Gas.
From unacademy.com
Notes on Formulas Involved With the Specific Heat Capacity of Gases What Is Heat Capacity Gas The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). here, we focus on the heat capacity with the volume held constant. Its si. What Is Heat Capacity Gas.
From www.youtube.com
Constant Volume Heating of a Gas YouTube What Is Heat Capacity Gas the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; When we investigate the energy. We can calculate it for an ideal gas. Its si unit is j k. The heat capacity of a. What Is Heat Capacity Gas.
From edumentors.co.uk
GCSE Physics Particle model of matter Edumentors What Is Heat Capacity Gas Its si unit is j k. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). When we investigate the energy. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Heat capacity of an. What Is Heat Capacity Gas.
From www.youtube.com
Thermodynamics 44 Ideal Gas Specific Heat example 1 YouTube What Is Heat Capacity Gas When we investigate the energy. if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\). Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c =. What Is Heat Capacity Gas.
From www.researchgate.net
Temperature dependent coefficients of ideal gas heat capacity equation What Is Heat Capacity Gas the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. When we investigate the energy. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. here, we focus on the heat capacity with the volume held constant. Calculate the. What Is Heat Capacity Gas.
From www.slideserve.com
PPT The Heat Capacity of a Diatomic Gas PowerPoint Presentation, free What Is Heat Capacity Gas the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. Heat capacity of an ideal. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. define heat capacity. What Is Heat Capacity Gas.
From www.slideshare.net
Heat Capacity What Is Heat Capacity Gas if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\). here, we focus on the heat capacity with the volume held constant. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. When. What Is Heat Capacity Gas.
From memim.com
Heat capacity ratio What Is Heat Capacity Gas here, we focus on the heat capacity with the volume held constant. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). Its si unit is j k. if we heat or do work on any gas—real or ideal—the energy change is. What Is Heat Capacity Gas.
From www.youtube.com
Constant Pressure Heating of a gas YouTube What Is Heat Capacity Gas Heat capacity of an ideal. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; define heat capacity of an ideal gas for a specific process; the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. The heat capacity of a body is. What Is Heat Capacity Gas.
From www.slideserve.com
PPT THERMODYNAMIC PowerPoint Presentation, free download ID6381537 What Is Heat Capacity Gas When we investigate the energy. We can calculate it for an ideal gas. here, we focus on the heat capacity with the volume held constant. define heat capacity of an ideal gas for a specific process; Its si unit is j k. if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\).. What Is Heat Capacity Gas.
From studylib.net
Heat Capacity of Metals PreLab What Is Heat Capacity Gas The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. define heat capacity of an ideal gas for a specific. What Is Heat Capacity Gas.
From www.geeksforgeeks.org
Heat Capacity Definition, Formula, Unit, Examples, FAQs What Is Heat Capacity Gas the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. if we heat or do work on any. What Is Heat Capacity Gas.
From www.slideserve.com
PPT Specific heat of gases PowerPoint Presentation, free download What Is Heat Capacity Gas if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\). the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. here, we focus on the heat capacity with the volume held constant. When. What Is Heat Capacity Gas.
From www.slideserve.com
PPT Chapter 9 Energy, Enthalpy and Thermochemistry PowerPoint What Is Heat Capacity Gas We can calculate it for an ideal gas. if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\). Heat capacity of an ideal. Its si unit is j k. When we investigate the energy. Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; define heat capacity of. What Is Heat Capacity Gas.
From byjus.com
One mole of an ideal gas whose pressure changes with volume as P=αV What Is Heat Capacity Gas in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. the heat capacity (c) of a body of matter is the quantity. What Is Heat Capacity Gas.
From www.slideserve.com
PPT Heat capacity and Specific Heat PowerPoint Presentation, free What Is Heat Capacity Gas We can calculate it for an ideal gas. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\). The heat capacity of a body is the quantity of heat required to raise its. What Is Heat Capacity Gas.
From www.youtube.com
19.7 Heat Capacities of an Ideal Gas YouTube What Is Heat Capacity Gas the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. if we heat or do work on any gas—real or ideal—the energy change is \(e=q+w\). in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c. What Is Heat Capacity Gas.
From www.grc.nasa.gov
Specific Heats Calorically Imperfect Gas What Is Heat Capacity Gas Heat capacity of an ideal. When we investigate the energy. Its si unit is j k. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. What Is Heat Capacity Gas.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free What Is Heat Capacity Gas Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. When we investigate the energy.. What Is Heat Capacity Gas.
From www.slideserve.com
PPT Chapter 17 The first law of thermodynamics PowerPoint What Is Heat Capacity Gas define heat capacity of an ideal gas for a specific process; Calculate the specific heat of an ideal gas for either an isobaric or isochoric process; We can calculate it for an ideal gas. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. in the chapter on temperature. What Is Heat Capacity Gas.