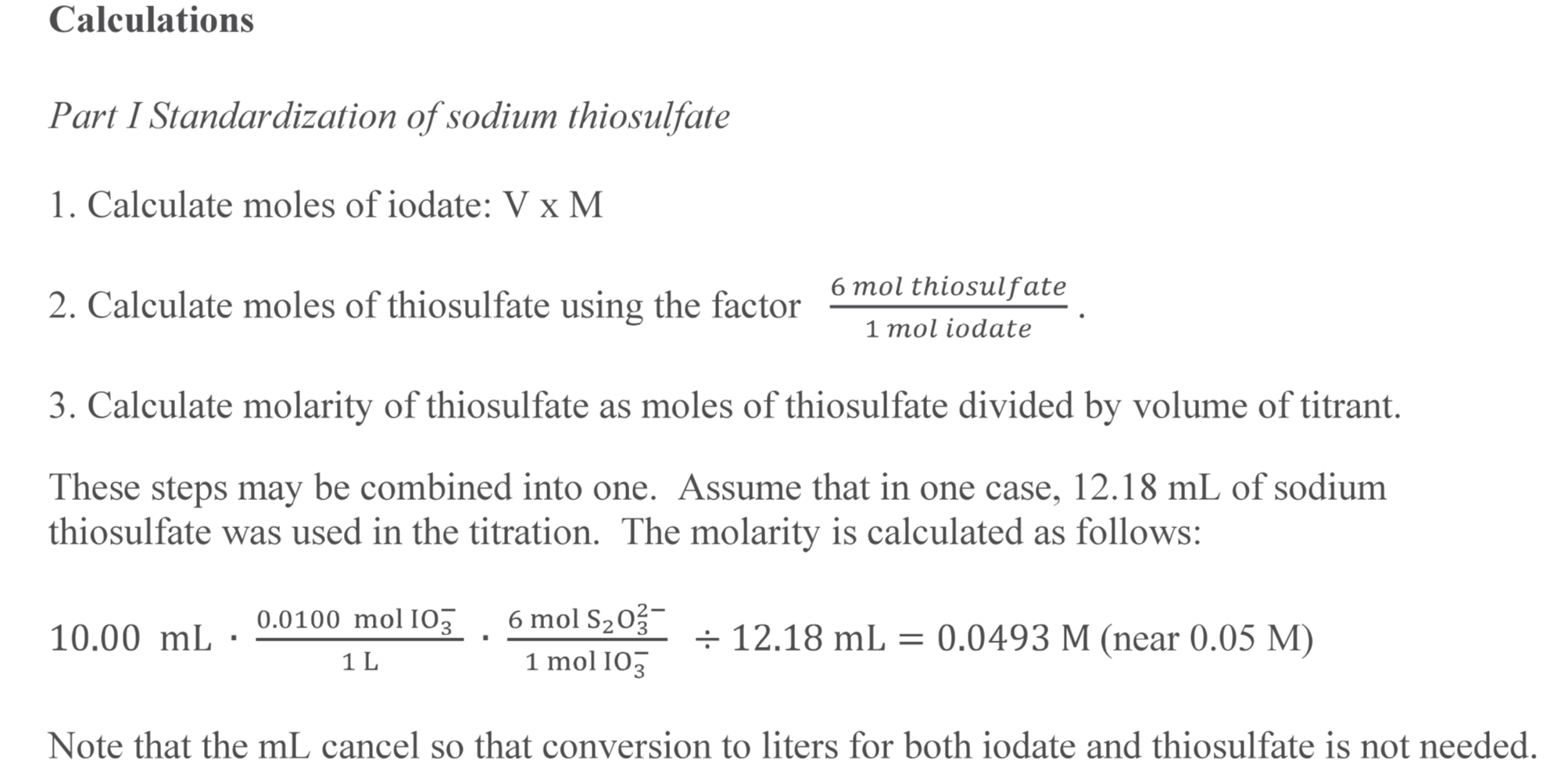
Copper Iodide And Sodium Thiosulfate Equation . An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. The amount of copper in brass may be found by converting the brass sample to a nearly neutral. The iodometric determination of copper in brass. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. The initial reaction, not the titration,. In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. You can find the amount of iodine liberated by titration with sodium thiosulphate solution.
from kadenqwoconnor.blogspot.com
As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass. An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. The initial reaction, not the titration,. The iodometric determination of copper in brass. The amount of copper in brass may be found by converting the brass sample to a nearly neutral. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. You can find the amount of iodine liberated by titration with sodium thiosulphate solution.
standardize sodium thiosulfate
Copper Iodide And Sodium Thiosulfate Equation You can find the amount of iodine liberated by titration with sodium thiosulphate solution. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. The iodometric determination of copper in brass. In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass. An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. The initial reaction, not the titration,. The amount of copper in brass may be found by converting the brass sample to a nearly neutral. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. You can find the amount of iodine liberated by titration with sodium thiosulphate solution.
From www.numerade.com
Aqueous copper(II) ion reacts with aqueous iodide ion to yield solid Copper Iodide And Sodium Thiosulfate Equation An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. The amount of copper in brass may be found by converting the brass sample to a nearly neutral. You can find the amount of iodine liberated by titration with sodium thiosulphate solution.. Copper Iodide And Sodium Thiosulfate Equation.
From www.numerade.com
Lab 8 Preparation and Standardization of Sodium Thiosulfate (Na2S2O3 Copper Iodide And Sodium Thiosulfate Equation The initial reaction, not the titration,. As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. The iodometric determination of copper in brass. The amount of copper in brass may be found. Copper Iodide And Sodium Thiosulfate Equation.
From www.dreamstime.com
Vector Skeletal Formula of Sodium Thiosulfate. Drug Chemical Mol Stock Copper Iodide And Sodium Thiosulfate Equation As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. The initial reaction, not the titration,. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. In this. Copper Iodide And Sodium Thiosulfate Equation.
From www.chegg.com
Solved 2. Potassium iodate and sodium thiosulphate react Copper Iodide And Sodium Thiosulfate Equation The amount of copper in brass may be found by converting the brass sample to a nearly neutral. The iodometric determination of copper in brass. In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass. You can find the amount of iodine liberated by titration. Copper Iodide And Sodium Thiosulfate Equation.
From testbook.com
Sodium Thiosulfate Introduction, Properties, Formula, Structure Copper Iodide And Sodium Thiosulfate Equation The iodometric determination of copper in brass. In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass. The initial reaction, not the titration,. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. You. Copper Iodide And Sodium Thiosulfate Equation.
From in.pinterest.com
What is the formula of sodium thiosulphate? Solubility, Sodium, Chemistry Copper Iodide And Sodium Thiosulfate Equation The amount of copper in brass may be found by converting the brass sample to a nearly neutral. An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. You can find the amount of iodine liberated by titration with sodium thiosulphate solution.. Copper Iodide And Sodium Thiosulfate Equation.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Copper Iodide And Sodium Thiosulfate Equation An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. The iodometric determination of copper in brass. You can find the. Copper Iodide And Sodium Thiosulfate Equation.
From www.toppr.com
.48 Sodium thiosulphate (Na2S2O3 5H20) reacts with iodine as follows Copper Iodide And Sodium Thiosulfate Equation An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get. Copper Iodide And Sodium Thiosulfate Equation.
From www.youtube.com
Iodometric Estimation of Copper using Sodium thiosulphate YouTube Copper Iodide And Sodium Thiosulfate Equation An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. The iodometric determination of copper in brass. The amount of copper. Copper Iodide And Sodium Thiosulfate Equation.
From www.slideshare.net
Writing More Complex Redox Equations Copper Iodide And Sodium Thiosulfate Equation In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass. The amount of copper in brass may be found by converting the brass sample to a nearly neutral. An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to. Copper Iodide And Sodium Thiosulfate Equation.
From byjus.com
Oxidation state of sulphur in sodium thiosulphate during the reaction Copper Iodide And Sodium Thiosulfate Equation An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized. Copper Iodide And Sodium Thiosulfate Equation.
From stock.adobe.com
Sodium thiosulfate, chemical structure. Skeletal formula. vector de Copper Iodide And Sodium Thiosulfate Equation In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. The initial reaction, not the titration,. As the sodium thiosulphate solution is run in. Copper Iodide And Sodium Thiosulfate Equation.
From www.chegg.com
Solved Name of the Experiment Estimation of copper in the Copper Iodide And Sodium Thiosulfate Equation An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. The iodometric determination of copper in brass. As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. You can find the amount of iodine liberated. Copper Iodide And Sodium Thiosulfate Equation.
From www.youtube.com
Thiosulfate YouTube Copper Iodide And Sodium Thiosulfate Equation Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass. The initial reaction, not the titration,. You can find the amount of iodine liberated. Copper Iodide And Sodium Thiosulfate Equation.
From www.youtube.com
How to Write the Formula for Sodium thiosulfate YouTube Copper Iodide And Sodium Thiosulfate Equation As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. The iodometric determination of copper in brass. In this experiment, iodometric titration using a standardized. Copper Iodide And Sodium Thiosulfate Equation.
From www.youtube.com
OCR A level Chemisty Unit F325 Module 3 Redox Titrations Using Copper Iodide And Sodium Thiosulfate Equation You can find the amount of iodine liberated by titration with sodium thiosulphate solution. As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. The amount of copper in brass may be found by converting the brass sample to a nearly neutral. You can find the amount of iodine liberated by titration with. Copper Iodide And Sodium Thiosulfate Equation.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Copper Iodide And Sodium Thiosulfate Equation You can find the amount of iodine liberated by titration with sodium thiosulphate solution. The iodometric determination of copper in brass. As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. The initial reaction, not the titration,. In this. Copper Iodide And Sodium Thiosulfate Equation.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation ID5408111 Copper Iodide And Sodium Thiosulfate Equation In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass. The iodometric determination of copper in brass. An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the.. Copper Iodide And Sodium Thiosulfate Equation.
From www.chegg.com
Solved + + 2. Potassium iodate and sodium thiosulphate react Copper Iodide And Sodium Thiosulfate Equation You can find the amount of iodine liberated by titration with sodium thiosulphate solution. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. The initial reaction, not the titration,. The amount of copper in. Copper Iodide And Sodium Thiosulfate Equation.
From www.slideserve.com
PPT Booklet to help with Unit 27 PowerPoint Presentation ID1951670 Copper Iodide And Sodium Thiosulfate Equation As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. You. Copper Iodide And Sodium Thiosulfate Equation.
From www.youtube.com
Iodine and sodium thiosulfate titrations YouTube Copper Iodide And Sodium Thiosulfate Equation The initial reaction, not the titration,. The iodometric determination of copper in brass. As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. The amount. Copper Iodide And Sodium Thiosulfate Equation.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Copper Iodide And Sodium Thiosulfate Equation Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. The initial reaction, not the titration,. The amount of copper in brass may be found by converting the brass sample to a nearly neutral. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. The. Copper Iodide And Sodium Thiosulfate Equation.
From www.numerade.com
a solution of sodium thiosulfate was standardized by dissolving 01110 g Copper Iodide And Sodium Thiosulfate Equation In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass. The iodometric determination of copper in brass. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. The amount of copper in brass may. Copper Iodide And Sodium Thiosulfate Equation.
From www.numerade.com
SOLVED Case To standardize an unknown solution of sodium thiosulfate Copper Iodide And Sodium Thiosulfate Equation An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. The initial reaction, not the titration,. You can find the amount. Copper Iodide And Sodium Thiosulfate Equation.
From www.youtube.com
Eureka 2017 Iodometry Redox Titration KIO3 Vs Thiosulfate YouTube Copper Iodide And Sodium Thiosulfate Equation The iodometric determination of copper in brass. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. The initial reaction, not. Copper Iodide And Sodium Thiosulfate Equation.
From www.flinnsci.com
Rate of Reaction of Sodium Thiosulfate and Hydrochloric Acid Copper Iodide And Sodium Thiosulfate Equation As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. The amount of copper in brass may be found by converting the brass sample to a nearly neutral. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. An iodometric redox titration using sodium thiosulfate solution and a starch. Copper Iodide And Sodium Thiosulfate Equation.
From kadenqwoconnor.blogspot.com
standardize sodium thiosulfate Copper Iodide And Sodium Thiosulfate Equation The initial reaction, not the titration,. As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. An iodometric. Copper Iodide And Sodium Thiosulfate Equation.
From www.youtube.com
Iodine and sodium thiosulfate redox titration calculations ALevel Copper Iodide And Sodium Thiosulfate Equation In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass. The amount of copper in brass may be found by converting the brass sample to a nearly neutral. The iodometric determination of copper in brass. You can find the amount of iodine liberated by titration. Copper Iodide And Sodium Thiosulfate Equation.
From www.coursehero.com
[Solved] Standardization of Sodium Thiosulphate Stock Solution Copper Iodide And Sodium Thiosulfate Equation The iodometric determination of copper in brass. The initial reaction, not the titration,. In this experiment, iodometric titration using a standardized 0 m sodium thiosulfate solution is utilized to determine the percent of copper in an unknown brass. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. As. Copper Iodide And Sodium Thiosulfate Equation.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Copper Iodide And Sodium Thiosulfate Equation The initial reaction, not the titration,. An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. The amount of copper in. Copper Iodide And Sodium Thiosulfate Equation.
From www.slideserve.com
PPT REACTION PREDICTION PreAP CHEMISTRY PowerPoint Presentation Copper Iodide And Sodium Thiosulfate Equation You can find the amount of iodine liberated by titration with sodium thiosulphate solution. As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. The iodometric determination of copper in brass. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. In. Copper Iodide And Sodium Thiosulfate Equation.
From www.dreamstime.com
3D Image of Sodium Thiosulfate Skeletal Formula Stock Illustration Copper Iodide And Sodium Thiosulfate Equation You can find the amount of iodine liberated by titration with sodium thiosulphate solution. The iodometric determination of copper in brass. An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. Iodometric determination of copper is based on the oxidation of iodides. Copper Iodide And Sodium Thiosulfate Equation.
From www.slideserve.com
PPT Preadjustment of analyte oxidation state PowerPoint Presentation Copper Iodide And Sodium Thiosulfate Equation The initial reaction, not the titration,. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the. Copper Iodide And Sodium Thiosulfate Equation.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Copper Iodide And Sodium Thiosulfate Equation As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii) ions, which get reduced to $\ce{cu+}$. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. In this experiment, iodometric titration using a standardized. Copper Iodide And Sodium Thiosulfate Equation.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Copper Iodide And Sodium Thiosulfate Equation As the sodium thiosulphate solution is run in from a burette, the colour of the iodine fades. An iodometric redox titration using sodium thiosulfate solution and a starch indicator can then be conducted to calculate the concentration of iodine formed [3] and hence, indirectly, the. Iodometric determination of copper is based on the oxidation of iodides to iodine by copper(ii). Copper Iodide And Sodium Thiosulfate Equation.