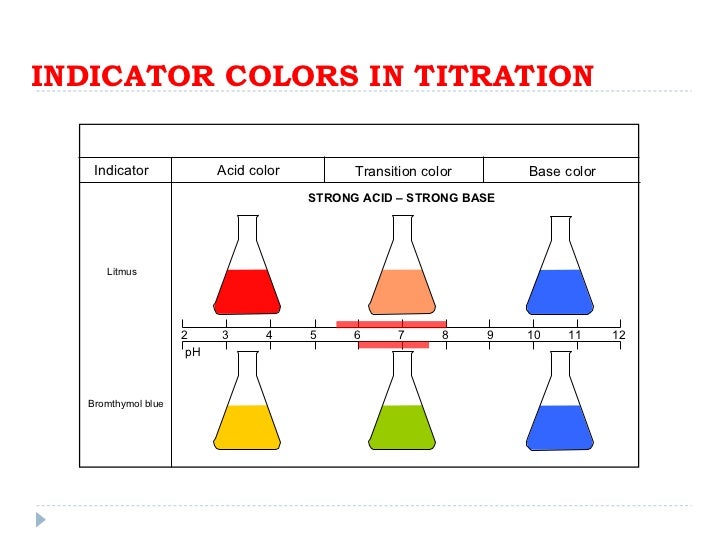
Titration For Indicators . titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. Remember that the equivalence point of a titration is where you have mixed the two substances. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. choosing indicators for titrations. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. The graph shows the results obtained using. choosing indicators for titrations. in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end.
from mungfali.com
titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. choosing indicators for titrations. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. The graph shows the results obtained using. Remember that the equivalence point of a titration is where you have mixed the two substances. in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. choosing indicators for titrations.
Acid Base Titration Indicator
Titration For Indicators revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. The graph shows the results obtained using. choosing indicators for titrations. Remember that the equivalence point of a titration is where you have mixed the two substances. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. choosing indicators for titrations.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration For Indicators choosing indicators for titrations. in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. Remember that the equivalence point of a titration is where you have mixed the. Titration For Indicators.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Titration For Indicators Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. Remember that the equivalence point of a titration is where you have mixed the two substances.. Titration For Indicators.
From exogrvkja.blob.core.windows.net
Indicators For Titrations at Leslie Jackson blog Titration For Indicators in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. choosing indicators for titrations. Remember that the equivalence point of a titration. Titration For Indicators.
From dxovrotfq.blob.core.windows.net
Titration Of Indicators at Beverly Estrada blog Titration For Indicators The graph shows the results obtained using. choosing indicators for titrations. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. choosing indicators for titrations. titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete,. Titration For Indicators.
From exogrvkja.blob.core.windows.net
Indicators For Titrations at Leslie Jackson blog Titration For Indicators titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. The graph shows the results obtained using. in the. Titration For Indicators.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Titration For Indicators Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. The graph shows the results obtained using. Remember that the equivalence point of a titration is where you have. Titration For Indicators.
From dxozxirwd.blob.core.windows.net
Chemical Indicator In A Titration at Nicholas Terrell blog Titration For Indicators titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. Remember that the equivalence point of a titration is where you have mixed the two substances. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation.. Titration For Indicators.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Titration For Indicators titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. The graph shows the results obtained using. in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. choosing indicators for titrations. . Titration For Indicators.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Titration For Indicators Remember that the equivalence point of a titration is where you have mixed the two substances. The graph shows the results obtained using. choosing indicators for titrations. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Remember that the equivalence point of a. Titration For Indicators.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Titration For Indicators The graph shows the results obtained using. choosing indicators for titrations. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. choosing indicators for titrations. Remember that the equivalence point of a titration is where you have mixed the two substances. titration, also known as volumetric analysis, is. Titration For Indicators.
From dxoqotyly.blob.core.windows.net
Acid Base Titration Indicator Example at Amanda Byrne blog Titration For Indicators in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. choosing indicators for titrations. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. choosing indicators for titrations. Remember that the. Titration For Indicators.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive Titration For Indicators titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. Remember that the equivalence point of a titration is where you have mixed the two substances.. Titration For Indicators.
From www.slideshare.net
Acid base titration Titration For Indicators The graph shows the results obtained using. Remember that the equivalence point of a titration is where you have mixed the two substances. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. titration, also known as volumetric analysis, is a method in which. Titration For Indicators.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Titration For Indicators revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. choosing indicators for titrations. Remember that the equivalence point of a titration is where you have mixed the two substances. choosing indicators for titrations. Remember that the equivalence point of a titration is. Titration For Indicators.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titration For Indicators choosing indicators for titrations. The graph shows the results obtained using. in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. Remember that the equivalence point of a titration is where you have mixed the two substances. Remember that the equivalence point of a titration is where. Titration For Indicators.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration For Indicators titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. in the strong acid titration, use of any of the three indicators should yield reasonably. Titration For Indicators.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration For Indicators Remember that the equivalence point of a titration is where you have mixed the two substances. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. The graph shows the results obtained using. choosing indicators for titrations. in the strong acid titration, use of any of the three indicators. Titration For Indicators.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog Titration For Indicators Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. The graph shows the results obtained using. Remember that the equivalence point of a titration is. Titration For Indicators.
From www.science-revision.co.uk
Titrations Titration For Indicators choosing indicators for titrations. The graph shows the results obtained using. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. titration, also known. Titration For Indicators.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Titration For Indicators in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. The graph shows the results obtained using. titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. choosing indicators for titrations. . Titration For Indicators.
From www.youtube.com
Acids and Bases Choosing an Indicator for Titration YouTube Titration For Indicators in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. Remember that the equivalence point of a titration is where you have mixed the two substances. titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction. Titration For Indicators.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID2134619 Titration For Indicators choosing indicators for titrations. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. The graph shows the results obtained using. choosing indicators for titrations. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my. Titration For Indicators.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration For Indicators titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. choosing indicators for titrations. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. choosing indicators for titrations.. Titration For Indicators.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Titration For Indicators choosing indicators for titrations. choosing indicators for titrations. in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. The graph shows. Titration For Indicators.
From wisc.pb.unizin.org
D30.1 AcidBase Indicators Chemistry 109 Fall 2021 Titration For Indicators Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. choosing indicators for titrations. titration, also known as volumetric analysis, is a method in which the titrant. Titration For Indicators.
From dxovrotfq.blob.core.windows.net
Titration Of Indicators at Beverly Estrada blog Titration For Indicators revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. The graph shows the results obtained using. titration, also known as volumetric. Titration For Indicators.
From theedge.com.hk
Chemistry How To Titration The Edge Titration For Indicators Remember that the equivalence point of a titration is where you have mixed the two substances. The graph shows the results obtained using. choosing indicators for titrations. choosing indicators for titrations. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. titration,. Titration For Indicators.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration For Indicators choosing indicators for titrations. choosing indicators for titrations. titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. Remember that the equivalence point of a titration is where you have mixed the two substances. The graph shows the results obtained using. in. Titration For Indicators.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Titration For Indicators The graph shows the results obtained using. choosing indicators for titrations. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. Remember that the equivalence point of a titration is where you have mixed the two substances. choosing indicators for titrations. titration, also known as volumetric analysis, is. Titration For Indicators.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration For Indicators Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. titration, also known as volumetric analysis, is a method in which the titrant is added from a burette. Titration For Indicators.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Titration For Indicators in the strong acid titration, use of any of the three indicators should yield reasonably sharp color changes and accurate end. The graph shows the results obtained using. choosing indicators for titrations. titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. . Titration For Indicators.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Titration For Indicators The graph shows the results obtained using. choosing indicators for titrations. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. Remember that the equivalence point of a titration is where you have mixed the two substances. choosing indicators for titrations. revision notes on 1.7.13 indicators used in. Titration For Indicators.
From mungfali.com
Acid Base Titration Indicator Titration For Indicators choosing indicators for titrations. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. titration, also known as volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and. choosing indicators for titrations.. Titration For Indicators.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Titration For Indicators Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. choosing indicators for titrations. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. choosing indicators for titrations. titration, also known as volumetric. Titration For Indicators.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration For Indicators choosing indicators for titrations. choosing indicators for titrations. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. titration, also known as volumetric. Titration For Indicators.