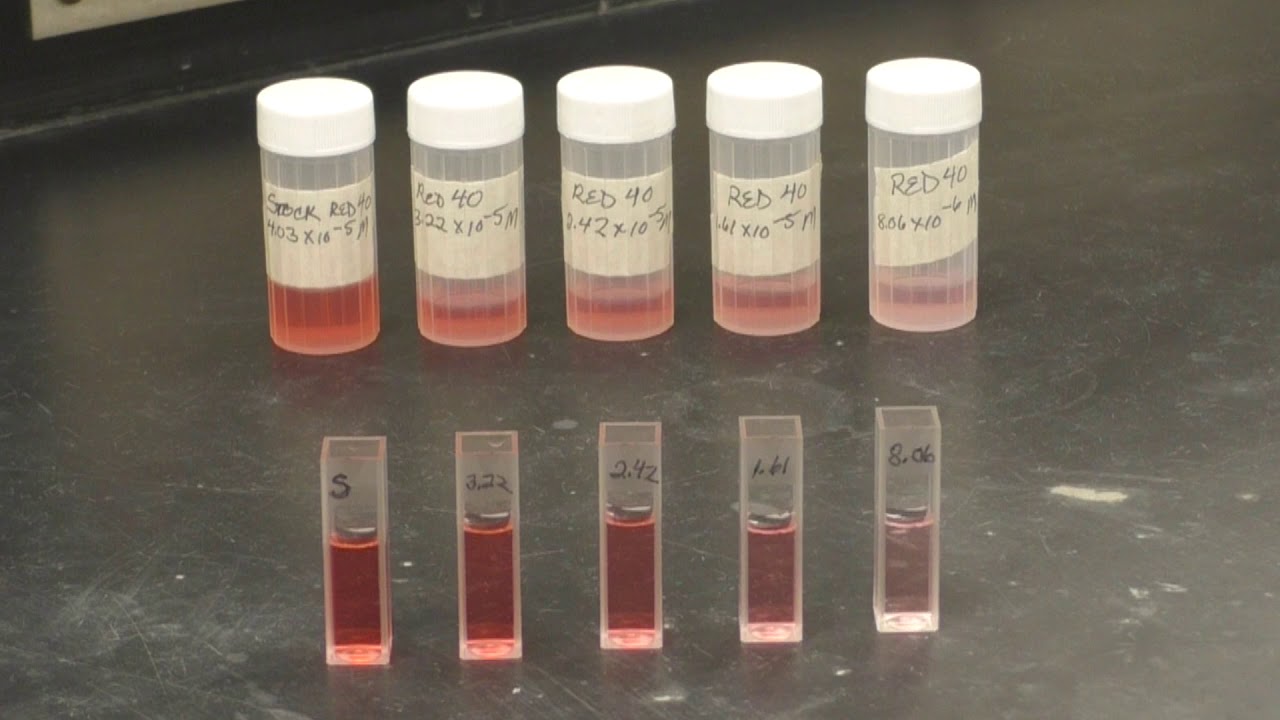
Beer's Law Application . Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer lambert law is the combined form of beer’s law and lambert’s law. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Spectrophotometry involves passing a beam of ultraviolet. Application of beer lambert law. Limitations of beer lambert law. Deviation of beer lambert law. Applications in physics and chemistry:
from www.youtube.com
Spectrophotometry involves passing a beam of ultraviolet. Deviation of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Application of beer lambert law. Beer lambert law is the combined form of beer’s law and lambert’s law. Limitations of beer lambert law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Applications in physics and chemistry: Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics.
Beer's Law and It's Application YouTube
Beer's Law Application Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Application of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Limitations of beer lambert law. Deviation of beer lambert law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Spectrophotometry involves passing a beam of ultraviolet. Beer lambert law is the combined form of beer’s law and lambert’s law. Applications in physics and chemistry:
From www.reddit.com
Visualization of Beer’s law in beer r/chemistry Beer's Law Application Applications in physics and chemistry: Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Spectrophotometry involves passing a beam of ultraviolet. Application of beer lambert law. Deviation of beer lambert law. Beer lambert law is the combined form of beer’s law and lambert’s law.. Beer's Law Application.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube Beer's Law Application Beer lambert law is the combined form of beer’s law and lambert’s law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Limitations of beer lambert law. Applications in physics and chemistry: Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Spectrophotometry involves passing a beam. Beer's Law Application.
From www.scribd.com
Application of Beer’s Law Quantitative analysis for a single analyte Beer's Law Application Deviation of beer lambert law. Applications in physics and chemistry: Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Beer lambert law is the combined form of beer’s law and lambert’s law. Limitations of beer lambert law. Application of beer lambert law. Spectrophotometry involves passing a beam of ultraviolet. Since the concentration, path. Beer's Law Application.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law Application Limitations of beer lambert law. Application of beer lambert law. Deviation of beer lambert law. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Chemistry uses beer’s law. Beer's Law Application.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Application Application of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer's Law Application.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer's Law Application Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Deviation of beer lambert law. Beer lambert law is the combined form of beer’s law and lambert’s law. Application. Beer's Law Application.
From pdfprof.com
La loi de Beer Lambert LHCE Beer's Law Application Applications in physics and chemistry: Spectrophotometry involves passing a beam of ultraviolet. Application of beer lambert law. Deviation of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which. Beer's Law Application.
From www.youtube.com
General Application of Beerlambert law YouTube Beer's Law Application Deviation of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Application of beer lambert law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer lambert law is the combined form of beer’s. Beer's Law Application.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer's Law Application Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Applications in physics and chemistry: Deviation of beer lambert law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer's Law Application.
From www.softpedia.com
Download Beer's Law Lab Beer's Law Application Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Application of beer lambert law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer lambert law is the combined form of beer’s law and lambert’s law. Deviation. Beer's Law Application.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer's Law Application Beer lambert law is the combined form of beer’s law and lambert’s law. Application of beer lambert law. Deviation of beer lambert law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law primarily finds applications in the field of spectrophotometry and the. Beer's Law Application.
From www.studypool.com
SOLUTION Beer Lambert's Law. applications and limitations? (Chemistry Beer's Law Application Spectrophotometry involves passing a beam of ultraviolet. Applications in physics and chemistry: Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Limitations of beer lambert law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer lambert. Beer's Law Application.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Application Spectrophotometry involves passing a beam of ultraviolet. Beer lambert law is the combined form of beer’s law and lambert’s law. Applications in physics and chemistry: Limitations of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Beer’s law primarily finds applications in the field of spectrophotometry and the study of. Beer's Law Application.
From chemistnotes.com
Beer lambert law Derivation, deviation, application, and limitations Beer's Law Application Spectrophotometry involves passing a beam of ultraviolet. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Limitations of beer lambert law. Application of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Deviation of beer lambert law. Since the concentration, path length and. Beer's Law Application.
From www.slideserve.com
PPT Introduction to Spectroscopic Methods of Analysis (part 2 Beer's Law Application Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Spectrophotometry involves passing a beam of ultraviolet. Deviation of beer lambert law. Beer lambert law is the combined form of beer’s law and lambert’s law. Applications in physics and chemistry: Application of beer lambert law.. Beer's Law Application.
From www.slideshare.net
Beers law Beer's Law Application Limitations of beer lambert law. Deviation of beer lambert law. Applications in physics and chemistry: Spectrophotometry involves passing a beam of ultraviolet. Beer lambert law is the combined form of beer’s law and lambert’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.. Beer's Law Application.
From www.youtube.com
beerlambert law best concept analytical chemistrychemmasters Beer's Law Application Beer lambert law is the combined form of beer’s law and lambert’s law. Application of beer lambert law. Spectrophotometry involves passing a beam of ultraviolet. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Deviation of beer lambert law. Chemistry uses beer’s law to. Beer's Law Application.
From www.slideserve.com
PPT Beers Law for a Single Component Sample PowerPoint Presentation Beer's Law Application Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Spectrophotometry involves passing a beam of ultraviolet. Limitations of beer lambert law. Applications in physics and chemistry: Beer lambert law is the combined form of beer’s law and lambert’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the. Beer's Law Application.
From studylib.net
Beer Lambert Law Beer's Law Application Application of beer lambert law. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Applications in physics and chemistry: Spectrophotometry involves passing a beam of ultraviolet. Deviation of beer lambert law. Since the concentration, path length and. Beer's Law Application.
From www.studypool.com
SOLUTION 2 beer lambert law complete Studypool Beer's Law Application Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Applications in physics and chemistry: Limitations of beer lambert law. Beer lambert law is the combined form of beer’s law and lambert’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. Beer's Law Application.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Application Limitations of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Applications in physics and chemistry: Beer lambert law is the combined form of beer’s law and lambert’s law. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Spectrophotometry involves passing a beam. Beer's Law Application.
From www.youtube.com
Beer's Law and It's Application YouTube Beer's Law Application Deviation of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Limitations of beer lambert law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer's Law Application.
From www.studocu.com
Chem 181 4 Beer's Law Beer Lab Pre lab Determining the Beer's Law Application Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Deviation of beer lambert law. Application of beer lambert law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Limitations of beer lambert law. Spectrophotometry involves passing a. Beer's Law Application.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer's Law Application Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Deviation of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Limitations of beer lambert law. Beer lambert law is the combined form of beer’s law and lambert’s law. Application of beer lambert law.. Beer's Law Application.
From www.youtube.com
Easy derivation of Lambert Beer Law UV Spectroscopy BSc MSc NET Beer's Law Application Applications in physics and chemistry: Spectrophotometry involves passing a beam of ultraviolet. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Limitations of beer lambert law. Since the concentration, path length and molar absorptivity are all directly. Beer's Law Application.
From byjus.com
BeerLambert Law Statement, Equation, Applications of BeerLambert Law Beer's Law Application Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Applications in physics and chemistry: Deviation of beer lambert law. Limitations of beer lambert law. Spectrophotometry involves passing a beam of ultraviolet. Application of beer lambert law. Beer. Beer's Law Application.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Application Beer lambert law is the combined form of beer’s law and lambert’s law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Deviation of beer lambert law. Spectrophotometry involves passing a beam of ultraviolet. Limitations of beer lambert law. Beer’s law primarily finds applications in the field of spectrophotometry and the study of. Beer's Law Application.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Application Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Deviation of beer lambert law. Application of beer lambert law. Beer lambert law is the combined form of beer’s law and lambert’s law. Limitations of beer lambert law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we. Beer's Law Application.
From www.studocu.com
Beer's Law Lab Beer's Law, 20192020 academic year Experiment 4 Beer's Law Application Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Deviation of beer lambert law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Limitations of beer lambert law. Applications in physics and chemistry: Beer lambert law is. Beer's Law Application.
From www.chegg.com
Solved SPECTROPHOTOMETRY. APPLY BEER'S LAW TO DETERMINE Beer's Law Application Application of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Spectrophotometry involves passing a beam of ultraviolet. Applications in physics and chemistry: Limitations of beer lambert law. Since the concentration, path length and. Beer's Law Application.
From www.slideshare.net
Beers law Beer's law Beer's Law Application Spectrophotometry involves passing a beam of ultraviolet. Limitations of beer lambert law. Deviation of beer lambert law. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Applications in physics and chemistry: Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which. Beer's Law Application.
From www.chegg.com
Solved BeerLambert Law Application Example 1 A compound Beer's Law Application Limitations of beer lambert law. Spectrophotometry involves passing a beam of ultraviolet. Beer lambert law is the combined form of beer’s law and lambert’s law. Beer’s law primarily finds applications in the field of spectrophotometry and the study of wave optics. Applications in physics and chemistry: Application of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research. Beer's Law Application.
From www.studocu.com
Beer's Law Experiment Beer’s Law and Spectrophotometry Purpose To Beer's Law Application Application of beer lambert law. Beer lambert law is the combined form of beer’s law and lambert’s law. Applications in physics and chemistry: Spectrophotometry involves passing a beam of ultraviolet. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Chemistry uses beer’s law to. Beer's Law Application.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Application Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Deviation of beer lambert law. Beer lambert law is the combined form of beer’s law and lambert’s law. Application of beer lambert law. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine. Beer's Law Application.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Application Applications in physics and chemistry: Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Chemistry uses beer’s law to gauge polymer deterioration, research oxidation, and determine the concentration of chemical. Spectrophotometry involves passing a beam of ultraviolet. Limitations of beer lambert law. Beer’s law. Beer's Law Application.