Calorimeter All Information . Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Chemists use calorimetry to determine the amount of heat. Technically, this is called an adiabatic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can create it at home using polystyrene cups.
from www.expii.com
Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Technically, this is called an adiabatic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can create it at home using polystyrene cups. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use calorimetry to determine the amount of heat. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings.
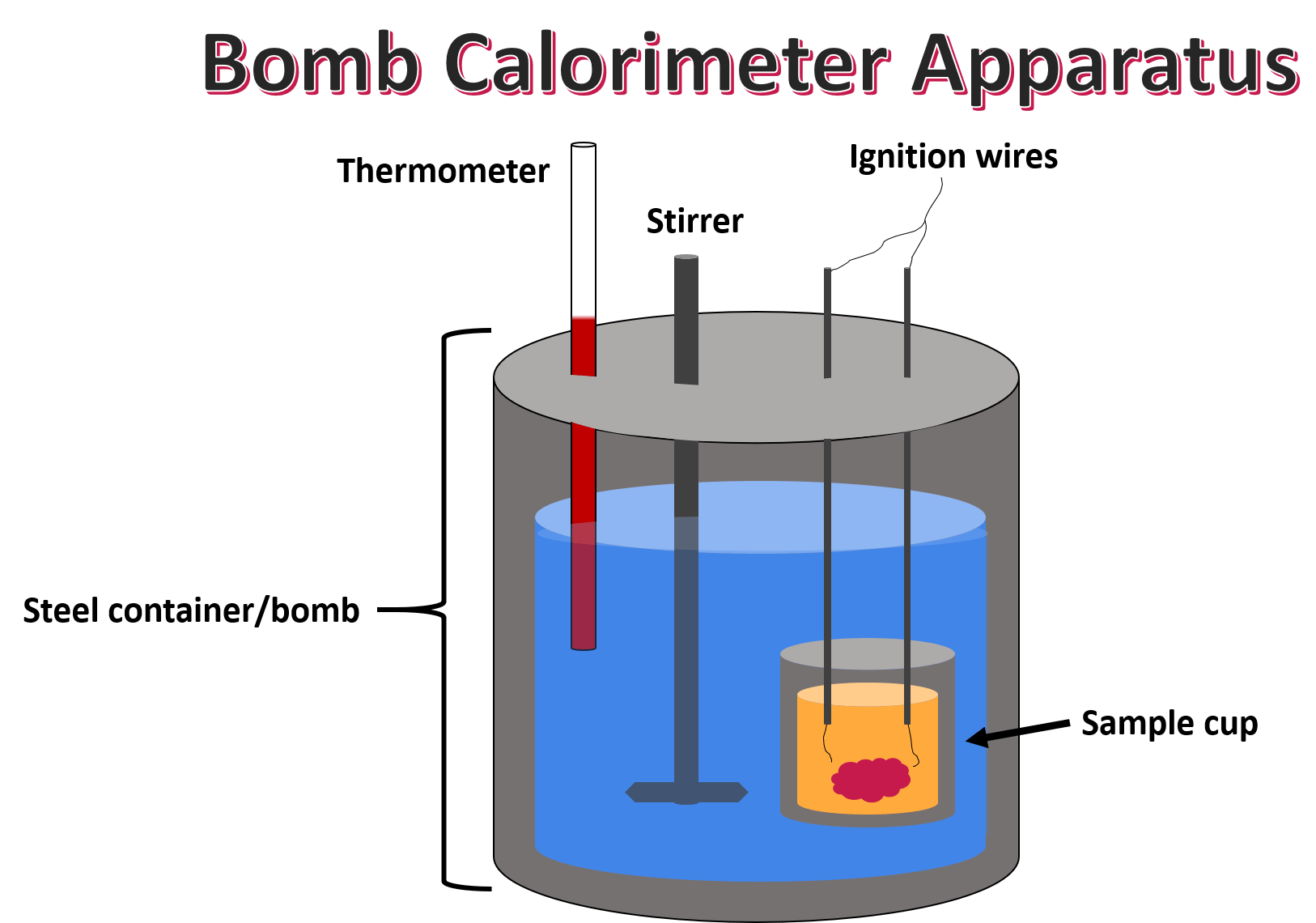
Bomb Calorimeter — Structure & Function Expii
Calorimeter All Information Chemists use calorimetry to determine the amount of heat. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Chemists use calorimetry to determine the amount of heat. Technically, this is called an adiabatic. A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can create it at home using polystyrene cups. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter All Information To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Chemists use calorimetry to determine the amount of heat. Technically, this is called an adiabatic. Calorimetry is a branch of science concerned with measuring a. Calorimeter All Information.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter All Information Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Chemists use calorimetry to determine. Calorimeter All Information.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter All Information To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use calorimetry to determine the amount of heat. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. A calorimeter measures the heat transferred to or from an object during a chemical or. Calorimeter All Information.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimeter All Information Chemists use calorimetry to determine the amount of heat. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal. Calorimeter All Information.
From www.slideserve.com
PPT An introduction to calorimeters for particle physics PowerPoint Calorimeter All Information A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can create it at home using polystyrene cups. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Chemists use calorimetry to determine the amount of heat. To determine the enthalpy,. Calorimeter All Information.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter All Information To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Technically, this is called an adiabatic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate. Calorimeter All Information.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter All Information A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter measures the heat transferred to or from an object during a chemical or. Calorimeter All Information.
From www.researchgate.net
Schematic representation of calorimeter system US4 at SKINR. Download Calorimeter All Information A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can create it at home using polystyrene cups. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change.. Calorimeter All Information.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter All Information Chemists use calorimetry to determine the amount of heat. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Technically, this is called an adiabatic. A calorimeter is a device that. Calorimeter All Information.
From www.scienceabc.com
How Do We Calculate How Many Carbs Are In The Food? Calorimeter All Information To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can create it at home using. Calorimeter All Information.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimeter All Information A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Technically, this is called an adiabatic. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use calorimetry to determine the amount of heat. Calorimetry is a branch of science concerned with measuring. Calorimeter All Information.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter All Information Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Chemists use calorimetry to determine the amount of heat. Technically, this is called an adiabatic.. Calorimeter All Information.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter All Information A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Technically, this is called an adiabatic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists. Calorimeter All Information.
From www.science-revision.co.uk
Calorimetry Calorimeter All Information Chemists use calorimetry to determine the amount of heat. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Technically, this is called an adiabatic.. Calorimeter All Information.
From www.researchgate.net
Measurement of heat using a chipbased microfluidic calorimeter. Data Calorimeter All Information Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use calorimetry to. Calorimeter All Information.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimeter All Information A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can create it at home using polystyrene cups. Calorimetry is a branch of science concerned with measuring a body’s state in terms of. Calorimeter All Information.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter All Information Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calorimetry is. Calorimeter All Information.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter All Information Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Chemists use calorimetry to determine the amount of heat. A calorimeter is a device that measures the heat flow produced. Calorimeter All Information.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeter All Information A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can create it at home using polystyrene cups. Technically, this is called an adiabatic. Calorimetry is a branch of science concerned with measuring. Calorimeter All Information.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter All Information Chemists use calorimetry to determine the amount of heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can create it at home using polystyrene cups. To determine the enthalpy, stability,. Calorimeter All Information.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter All Information Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter. Calorimeter All Information.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter All Information Technically, this is called an adiabatic. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can create it at home using polystyrene cups. A calorimeter is. Calorimeter All Information.
From www.laboratory-equipment.com
Laboratory Calorimeters from IKA Calorimeter All Information Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can create it at home using polystyrene cups. Calorimetry is a branch of science concerned with measuring a body’s state in terms. Calorimeter All Information.
From www.youtube.com
050 Calorimetry YouTube Calorimeter All Information A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Calorimeter All Information.
From users.highland.edu
Calorimetry Calorimeter All Information Chemists use calorimetry to determine the amount of heat. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. A calorimeter is a device that measures the heat flow. Calorimeter All Information.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter All Information Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use. Calorimeter All Information.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimeter All Information To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Technically, this is called an adiabatic. Chemists use calorimetry to determine the amount of heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter measures the heat transferred to or from an object. Calorimeter All Information.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimeter All Information Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry. Calorimeter All Information.
From saylordotorg.github.io
Calorimetry Calorimeter All Information A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can create it at home using polystyrene cups. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow. Calorimeter All Information.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter All Information Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical. Calorimeter All Information.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Calorimeter All Information To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Technically, this is called an adiabatic.. Calorimeter All Information.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter All Information Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Chemists use calorimetry to determine the amount of heat. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a. Calorimeter All Information.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimeter All Information A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Technically, this is called an adiabatic. Calorimetry is a field of thermochemistry that measures the amount of heat involved. Calorimeter All Information.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimeter All Information Technically, this is called an adiabatic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you can. Calorimeter All Information.
From www.linstitute.net
AQA A Level Biology复习笔记5.3.3 Biomass翰林国际教育 Calorimeter All Information Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device that measures the heat flow produced by a chemical reaction or. Calorimeter All Information.