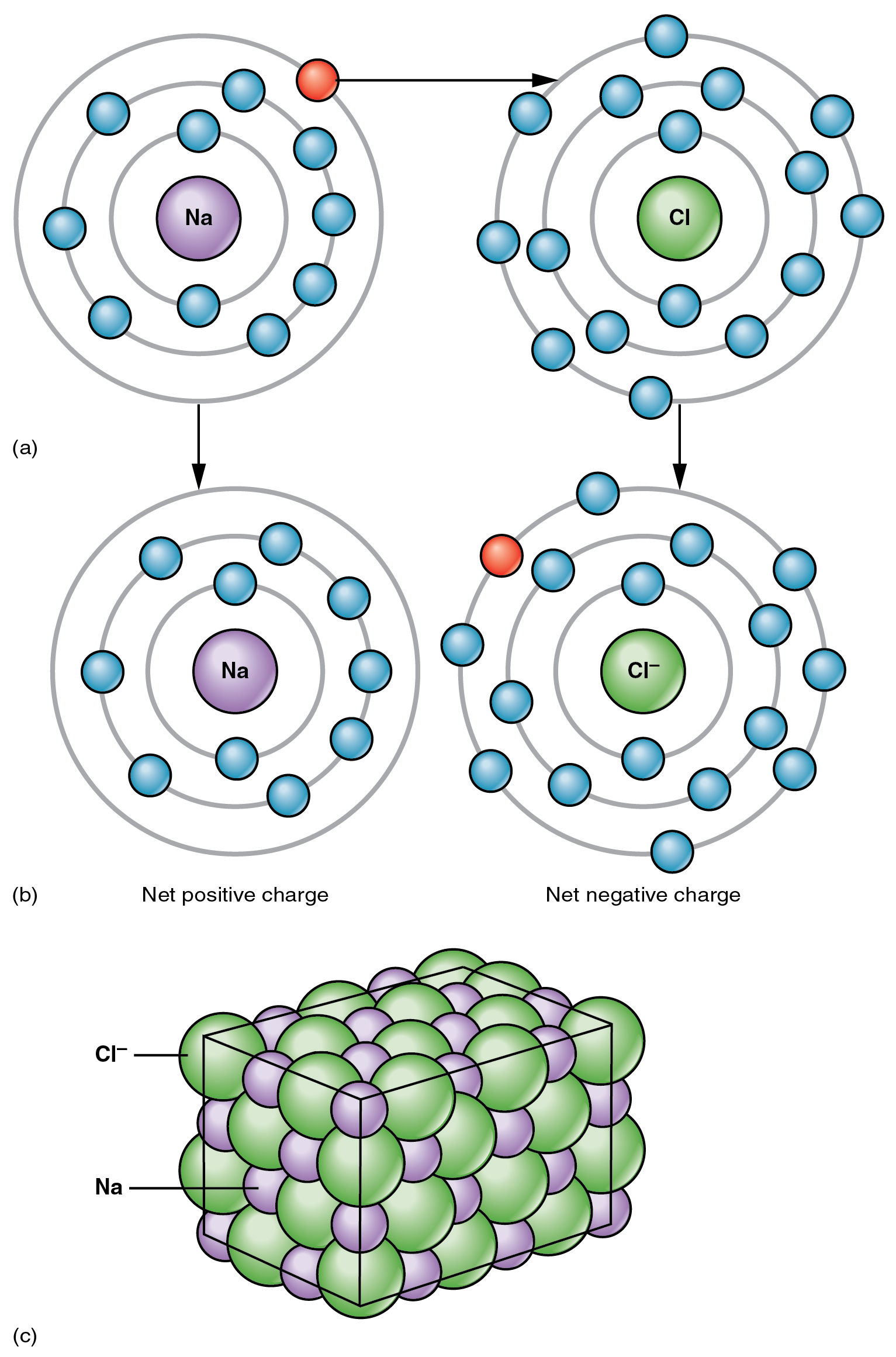
Chlorine Atom And Sodium . Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. The reaction between sodium and chlorine. Sodium is oxidized to sodium. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. For example, chlorine reacts with sodium: The reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties of a compound can be from its constituent elements. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: Chlorine has 7 electrons in its outer shell. When an atom of chlorine reacts it will gain one electron from. The diagrams show two ways of. It is in group 7 of the periodic table. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. Sodium + chlorine → sodium chloride. Original electron structure of sodium and chlorine atoms.
from oerpub.github.io
2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. Sodium + chlorine → sodium chloride. The reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties of a compound can be from its constituent elements. The diagrams show two ways of. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: For example, chlorine reacts with sodium: When an atom of chlorine reacts it will gain one electron from. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. Original electron structure of sodium and chlorine atoms. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms.
The top panel of this figure shows the orbit model of a sodium atom and
Chlorine Atom And Sodium The diagrams show two ways of. For example, chlorine reacts with sodium: It is in group 7 of the periodic table. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: The reaction between sodium and chlorine. When an atom of chlorine reacts it will gain one electron from. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. Sodium + chlorine → sodium chloride. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. The diagrams show two ways of. Original electron structure of sodium and chlorine atoms. The reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties of a compound can be from its constituent elements. Chlorine has 7 electrons in its outer shell. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. Sodium is oxidized to sodium.
From www.science-revision.co.uk
Polar bonds and molecules Chlorine Atom And Sodium It is in group 7 of the periodic table. The reaction between sodium and chlorine. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. Sodium + chlorine → sodium chloride. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: Sodium, a very reactive metal which. Chlorine Atom And Sodium.
From www.pikpng.com
Download Png Royalty Free Drawing Atoms Sodium Covalent Bonding Of Chlorine Atom And Sodium It is in group 7 of the periodic table. Chlorine has 7 electrons in its outer shell. Sodium + chlorine → sodium chloride. The reaction between sodium and chlorine. The reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties of a compound can be from its constituent elements. For example, chlorine reacts with. Chlorine Atom And Sodium.
From stock.adobe.com
Ionic covalent bonds examples. Chemical structural models. Atoms Chlorine Atom And Sodium For example, chlorine reacts with sodium: Sodium + chlorine → sodium chloride. The reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties of a compound can be from its constituent elements. Original electron structure of sodium and chlorine atoms. The diagrams show two ways of. Sodium, a very reactive metal which reacts with. Chlorine Atom And Sodium.
From www.gettyimages.com
Illustration Of Sodium And Chlorine Atoms Bonding HighRes Vector Chlorine Atom And Sodium The diagrams show two ways of. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. It is in group 7 of the periodic table. Original electron structure of sodium and chlorine atoms. When an atom of chlorine reacts it will gain one electron from. The reaction between sodium and chlorine. For example,. Chlorine Atom And Sodium.
From gardenandplate.com
Molecules Chlorine Atom And Sodium Sodium is oxidized to sodium. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. For example, chlorine reacts with sodium: For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. When an atom of chlorine reacts it will gain one electron from. Chlorine has 7 electrons in its outer shell.. Chlorine Atom And Sodium.
From www.numerade.com
The sodium chloride has a crystal structure (a unit cell) as described Chlorine Atom And Sodium For example, chlorine reacts with sodium: For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. Sodium is oxidized to sodium. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. Sodium + chlorine → sodium chloride. Chlorine has 7 electrons in its outer shell. The reaction between. Chlorine Atom And Sodium.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Chlorine Atom And Sodium For example, chlorine reacts with sodium: Sodium is oxidized to sodium. Original electron structure of sodium and chlorine atoms. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. When an atom of chlorine reacts it will gain one electron from. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride,. Chlorine Atom And Sodium.
From material-properties.org
Sodium and Chlorine Comparison Properties Material Properties Chlorine Atom And Sodium Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: Chlorine has 7 electrons in its outer shell. Sodium is oxidized to sodium. Sodium + chlorine → sodium chloride. The diagrams show two ways. Chlorine Atom And Sodium.
From ar.inspiredpencil.com
Atomic Structure Of Sodium Chloride Chlorine Atom And Sodium The diagrams show two ways of. Sodium + chlorine → sodium chloride. Original electron structure of sodium and chlorine atoms. When an atom of chlorine reacts it will gain one electron from. For example, chlorine reacts with sodium: Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. For example, when sodium reacts. Chlorine Atom And Sodium.
From www.slideserve.com
PPT Chapter 6 Ionic Compounds PowerPoint Presentation, free Chlorine Atom And Sodium The diagrams show two ways of. It is in group 7 of the periodic table. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: Sodium + chlorine → sodium chloride. Chlorine has 7 electrons in. Chlorine Atom And Sodium.
From www.dreamstime.com
NaCl Sodium Chloride. Molecule with Sodium and Chlorine Atoms. 3d Chlorine Atom And Sodium Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. When an atom of chlorine reacts it will gain one electron from. The diagrams show two ways of. The reaction between sodium and chlorine. Original electron structure of sodium and chlorine atoms. Sodium + chlorine → sodium chloride. Use questions to draw together. Chlorine Atom And Sodium.
From byjus.com
Sodium Chloride Preparation, Properties, Structure & Uses Byju's Chlorine Atom And Sodium Original electron structure of sodium and chlorine atoms. When an atom of chlorine reacts it will gain one electron from. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: The reaction between sodium and chlorine. For example, chlorine reacts with sodium: Chlorine has 7 electrons in its outer shell. Sodium,. Chlorine Atom And Sodium.
From fionaatbender.blogspot.com
Electron Arrangement of Sodium Chloride FionaatBender Chlorine Atom And Sodium Original electron structure of sodium and chlorine atoms. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. Sodium is oxidized to sodium. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: Chlorine has 7 electrons in its outer shell. The reaction between sodium and. Chlorine Atom And Sodium.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Chlorine Atom And Sodium 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: The reaction between sodium and chlorine. Chlorine has 7 electrons in its outer shell. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium. Chlorine Atom And Sodium.
From www.alamy.com
Sodium Hydroxide and Sodium Chloride Molecular Model of Atom. Vector Chlorine Atom And Sodium Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: The diagrams show two ways of. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. Sodium + chlorine →. Chlorine Atom And Sodium.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Atom And Sodium The reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties of a compound can be from its constituent elements. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. For example, chlorine reacts with sodium: 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react. Chlorine Atom And Sodium.
From www.dreamstime.com
Anions and Cations for Example Sodium and Chlorine Atoms. Stock Vector Chlorine Atom And Sodium The diagrams show two ways of. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: The reaction between sodium and chlorine. The reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties of a compound can be from its constituent elements. When an atom. Chlorine Atom And Sodium.
From www.dreamstime.com
NaCl Sodium Chloride. Molecule with Sodium and Chlorine Atoms. 3d Chlorine Atom And Sodium Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: Chlorine has 7 electrons in its outer shell. The reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties of a compound can be from its constituent elements. Sodium + chlorine → sodium chloride. The. Chlorine Atom And Sodium.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Chlorine Atom And Sodium For example, chlorine reacts with sodium: Original electron structure of sodium and chlorine atoms. Sodium + chlorine → sodium chloride. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: When an atom of chlorine reacts it will gain one electron from. It is in group 7 of the periodic table.. Chlorine Atom And Sodium.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Atom And Sodium When an atom of chlorine reacts it will gain one electron from. Original electron structure of sodium and chlorine atoms. For example, chlorine reacts with sodium: For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of:. Chlorine Atom And Sodium.
From www.science-revision.co.uk
Ionic bond formation Chlorine Atom And Sodium Chlorine has 7 electrons in its outer shell. Original electron structure of sodium and chlorine atoms. The reaction between sodium and chlorine. For example, chlorine reacts with sodium: 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. The diagrams show two ways of. Sodium, a very reactive metal which reacts with chlorine gas and produces. Chlorine Atom And Sodium.
From www.mooramo.com
The Formation of Ionic Compounds From Atoms Mooramo Chlorine Atom And Sodium Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: For example, chlorine reacts with sodium: The reaction between sodium and chlorine. The reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties of a compound can be from its constituent elements. Chlorine has 7. Chlorine Atom And Sodium.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Chlorine Atom And Sodium Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: Original electron structure of sodium and chlorine atoms. Sodium + chlorine → sodium chloride. Sodium is oxidized to sodium. For example, chlorine reacts with sodium: For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. The. Chlorine Atom And Sodium.
From www.alamy.com
1950s ionization of sodium and chlorine atoms after reaching melting Chlorine Atom And Sodium Sodium is oxidized to sodium. When an atom of chlorine reacts it will gain one electron from. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. Chlorine has 7 electrons in its outer shell. The reaction between. Chlorine Atom And Sodium.
From circuitdataboattrains.z14.web.core.windows.net
Chlorine Atom Diagram Chlorine Atom And Sodium Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. The reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties of a compound can be from its constituent elements. For example, chlorine reacts with sodium: Use questions to draw together and summarise the explanation of the. Chlorine Atom And Sodium.
From oerpub.github.io
The top panel of this figure shows the orbit model of a sodium atom and Chlorine Atom And Sodium Chlorine has 7 electrons in its outer shell. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. It is in group 7 of the periodic table. The reaction between sodium and chlorine. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. When an atom of chlorine. Chlorine Atom And Sodium.
From www2.victoriacollege.edu
formation of ionic bonds Chlorine Atom And Sodium For example, chlorine reacts with sodium: Sodium + chlorine → sodium chloride. Sodium is oxidized to sodium. When an atom of chlorine reacts it will gain one electron from. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine. Chlorine Atom And Sodium.
From www.thesciencehive.co.uk
Bonding and Structure* — the science sauce Chlorine Atom And Sodium For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. The reaction between sodium and chlorine. For example, chlorine reacts with sodium: Sodium is oxidized to sodium. It is in group 7 of the periodic table. The diagrams show two ways of. When an atom of chlorine reacts it will gain one electron from. Sodium. Chlorine Atom And Sodium.
From askfilo.com
Compare (a) sodium atom and sodium ion (b) chlorine atom and chloride io.. Chlorine Atom And Sodium The reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties of a compound can be from its constituent elements. Sodium is oxidized to sodium. For example, chlorine reacts with sodium: It is in group 7 of the periodic table. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine. Chlorine Atom And Sodium.
From brainly.in
6.Draw the atomic structure of sodium atom and chlorine atom.(Atomic Chlorine Atom And Sodium It is in group 7 of the periodic table. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: Original electron structure of sodium and chlorine atoms. Sodium + chlorine → sodium chloride. Chlorine has 7. Chlorine Atom And Sodium.
From www.priyamstudycentre.com
Sodium Chloride (NaCl) Uses, Crystal Chlorine Atom And Sodium When an atom of chlorine reacts it will gain one electron from. Original electron structure of sodium and chlorine atoms. For example, chlorine reacts with sodium: The reaction between sodium and chlorine. It is in group 7 of the periodic table. Chlorine has 7 electrons in its outer shell. For example, when sodium reacts with chlorine, electrons transfer from sodium. Chlorine Atom And Sodium.
From www.alamy.com
Sodium Hydroxide and Sodium Chloride Molecular Model of Atom. Vector Chlorine Atom And Sodium Sodium + chlorine → sodium chloride. Sodium is oxidized to sodium. When an atom of chlorine reacts it will gain one electron from. It is in group 7 of the periodic table. Chlorine has 7 electrons in its outer shell. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. Original electron structure. Chlorine Atom And Sodium.
From www.gettyimages.ae
Illustration Of Sodium Atom And Chlorine Atom HighRes Vector Graphic Chlorine Atom And Sodium Sodium is oxidized to sodium. For example, chlorine reacts with sodium: For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. Sodium + chlorine → sodium chloride. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. 2na (s) + cl 2 (g) → 2nacl (s) sodium and. Chlorine Atom And Sodium.
From pngtree.com
3d Rendering Of Sodium Chloride Molecule With Sodium And Chlorine Atoms Chlorine Atom And Sodium For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. For example, chlorine reacts with sodium: Sodium is oxidized to sodium. The reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties of a compound can be from its constituent elements. Sodium, a very reactive metal which reacts with. Chlorine Atom And Sodium.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Atom And Sodium 2na (s) + cl 2 (g) → 2nacl (s) sodium and chlorine react vigorously. The reaction between sodium and chlorine. Sodium, a very reactive metal which reacts with chlorine gas and produces sodium chloride, the neutral salt. Use questions to draw together and summarise the explanation of the reaction of sodium and chlorine in terms of: The reaction between sodium. Chlorine Atom And Sodium.