How Do You Measure Gas In Chemistry . It can be measured using a barometer or manometer. For each concentration of hydrochloric acid, plot a graph to show: Pressure is defined as the force exerted per unit area; Four quantities must be known for a. Volume of gas (cm 3) on the vertical axis. A gas syringe (left) or trough (right) should be used to measure a volume of gas. This particular gas law is. The liquid is placed in the u shaped tube connected to the big. The volume of a gas may be calculated from its number of moles using: P gas = pressure of confined gas. Volume of gas = moles × v m. The syringe is more easily operated but has a greater. P a = atmospheric pressure, p hg = pressure of mercury column; Time (s) on the horizontal axis. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas.
from www.youtube.com
P gas = pressure of confined gas. Volume of gas = moles × v m. A gas syringe (left) or trough (right) should be used to measure a volume of gas. The volume of a gas may be calculated from its number of moles using: The syringe is more easily operated but has a greater. Four quantities must be known for a. For each concentration of hydrochloric acid, plot a graph to show: P a = atmospheric pressure, p hg = pressure of mercury column; Volume of gas (cm 3) on the vertical axis. Time (s) on the horizontal axis.
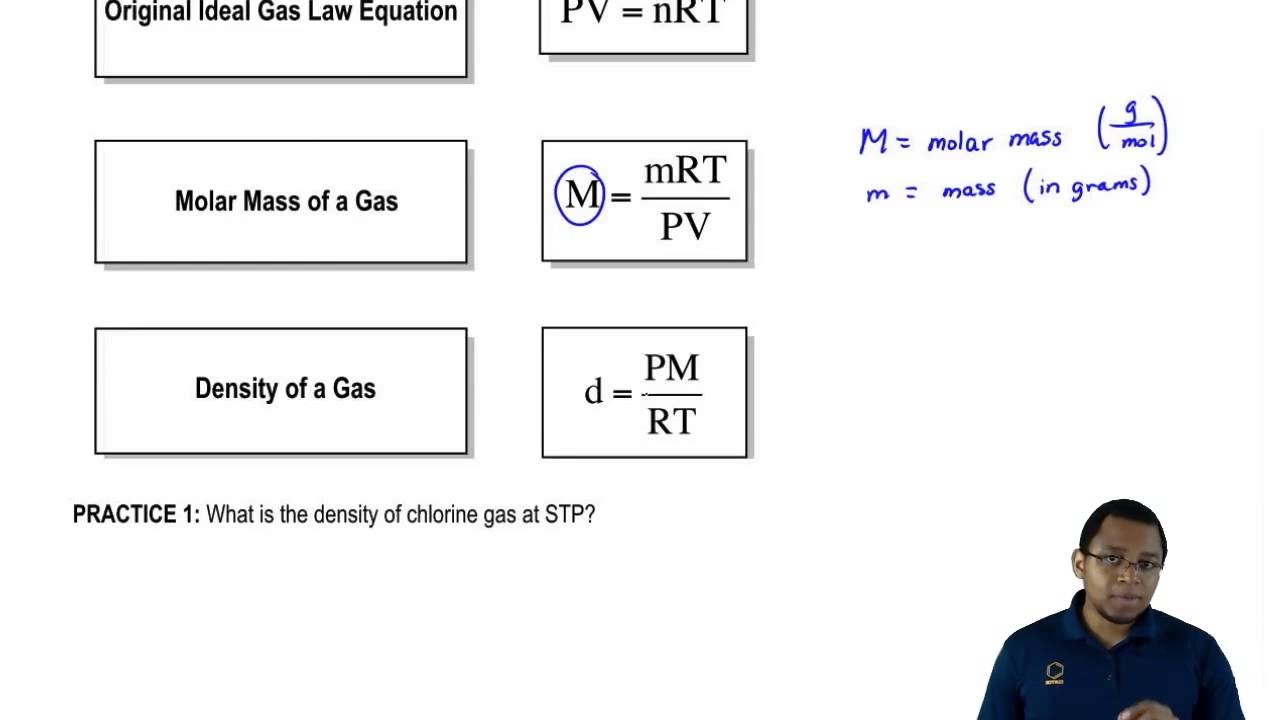
Using the Ideal Gas Law to find density or molar mass YouTube
How Do You Measure Gas In Chemistry Four quantities must be known for a. Volume of gas (cm 3) on the vertical axis. A gas syringe (left) or trough (right) should be used to measure a volume of gas. P gas = pressure of confined gas. Time (s) on the horizontal axis. Pressure is defined as the force exerted per unit area; The volume of a gas may be calculated from its number of moles using: A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. The syringe is more easily operated but has a greater. Four quantities must be known for a. Volume of gas = moles × v m. P a = atmospheric pressure, p hg = pressure of mercury column; The liquid is placed in the u shaped tube connected to the big. This particular gas law is. It can be measured using a barometer or manometer. For each concentration of hydrochloric acid, plot a graph to show:
From www.wikihow.com
3 Ways to Measure Density of Gases wikiHow How Do You Measure Gas In Chemistry A gas syringe (left) or trough (right) should be used to measure a volume of gas. P a = atmospheric pressure, p hg = pressure of mercury column; This particular gas law is. The syringe is more easily operated but has a greater. Volume of gas = moles × v m. The volume of a gas may be calculated from. How Do You Measure Gas In Chemistry.
From taylorwindam1952.blogspot.com
Taylor Windam How Do You Measure Volume Of A Gas How Do You Measure Gas In Chemistry Pressure is defined as the force exerted per unit area; The volume of a gas may be calculated from its number of moles using: The syringe is more easily operated but has a greater. Four quantities must be known for a. For each concentration of hydrochloric acid, plot a graph to show: A gas law is a simple mathematical formula. How Do You Measure Gas In Chemistry.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation, free download ID3496562 How Do You Measure Gas In Chemistry A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. A gas syringe (left) or trough (right) should be used to measure a volume of gas. P gas = pressure of confined gas. Pressure is defined as the force exerted per unit area; Time (s) on the horizontal axis. Volume. How Do You Measure Gas In Chemistry.
From www.youtube.com
How To Calculate Gas Volumes Chemical Calculations Chemistry How Do You Measure Gas In Chemistry P gas = pressure of confined gas. Four quantities must be known for a. Volume of gas (cm 3) on the vertical axis. Pressure is defined as the force exerted per unit area; Time (s) on the horizontal axis. This particular gas law is. The syringe is more easily operated but has a greater. P a = atmospheric pressure, p. How Do You Measure Gas In Chemistry.
From fyofvjcbv.blob.core.windows.net
How Do You Measure Gas Volume at John Courtney blog How Do You Measure Gas In Chemistry For each concentration of hydrochloric acid, plot a graph to show: P a = atmospheric pressure, p hg = pressure of mercury column; The syringe is more easily operated but has a greater. P gas = pressure of confined gas. Time (s) on the horizontal axis. This particular gas law is. A gas syringe (left) or trough (right) should be. How Do You Measure Gas In Chemistry.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID How Do You Measure Gas In Chemistry Volume of gas = moles × v m. For each concentration of hydrochloric acid, plot a graph to show: It can be measured using a barometer or manometer. A gas syringe (left) or trough (right) should be used to measure a volume of gas. P a = atmospheric pressure, p hg = pressure of mercury column; This particular gas law. How Do You Measure Gas In Chemistry.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation, free download ID3496562 How Do You Measure Gas In Chemistry For each concentration of hydrochloric acid, plot a graph to show: A gas syringe (left) or trough (right) should be used to measure a volume of gas. P gas = pressure of confined gas. Time (s) on the horizontal axis. Four quantities must be known for a. Pressure is defined as the force exerted per unit area; P a =. How Do You Measure Gas In Chemistry.
From www.slideserve.com
PPT Gas Volumes L.O. PowerPoint Presentation, free download ID5877069 How Do You Measure Gas In Chemistry Four quantities must be known for a. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. P a = atmospheric pressure, p hg = pressure of mercury column; This particular gas law is. For each concentration of hydrochloric acid, plot a graph to show: Time (s) on the horizontal. How Do You Measure Gas In Chemistry.
From www.youtube.com
Using the Ideal Gas Law to find density or molar mass YouTube How Do You Measure Gas In Chemistry The liquid is placed in the u shaped tube connected to the big. A gas syringe (left) or trough (right) should be used to measure a volume of gas. Volume of gas (cm 3) on the vertical axis. Volume of gas = moles × v m. A gas law is a simple mathematical formula that allows you to model, or. How Do You Measure Gas In Chemistry.
From www.showme.com
Ideal gas law example Science, Chemistry, Gases ShowMe How Do You Measure Gas In Chemistry The liquid is placed in the u shaped tube connected to the big. For each concentration of hydrochloric acid, plot a graph to show: It can be measured using a barometer or manometer. Four quantities must be known for a. A gas syringe (left) or trough (right) should be used to measure a volume of gas. A gas law is. How Do You Measure Gas In Chemistry.
From www.youtube.com
CHEMISTRY 101 Density of a gas, molar volume, and molar mass YouTube How Do You Measure Gas In Chemistry The syringe is more easily operated but has a greater. Time (s) on the horizontal axis. P gas = pressure of confined gas. Volume of gas (cm 3) on the vertical axis. It can be measured using a barometer or manometer. For each concentration of hydrochloric acid, plot a graph to show: This particular gas law is. Pressure is defined. How Do You Measure Gas In Chemistry.
From www.slideserve.com
PPT Natural Gas Measurement, Meters and Pipelines PowerPoint How Do You Measure Gas In Chemistry Four quantities must be known for a. The volume of a gas may be calculated from its number of moles using: Volume of gas (cm 3) on the vertical axis. It can be measured using a barometer or manometer. This particular gas law is. P a = atmospheric pressure, p hg = pressure of mercury column; For each concentration of. How Do You Measure Gas In Chemistry.
From www.youtube.com
Molar Mass of a Gas at STP Equations & Formulas, Chemistry Practice How Do You Measure Gas In Chemistry This particular gas law is. For each concentration of hydrochloric acid, plot a graph to show: A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. Volume of gas (cm 3) on the vertical axis. The liquid is placed in the u shaped tube connected to the big. Time (s). How Do You Measure Gas In Chemistry.
From www.youtube.com
Volume of 1 mole of any gas at STP using the ideal gas equation YouTube How Do You Measure Gas In Chemistry Time (s) on the horizontal axis. The syringe is more easily operated but has a greater. P a = atmospheric pressure, p hg = pressure of mercury column; A gas syringe (left) or trough (right) should be used to measure a volume of gas. Pressure is defined as the force exerted per unit area; Volume of gas = moles ×. How Do You Measure Gas In Chemistry.
From www.wikihow.com
3 Ways to Measure Density of Gases wikiHow How Do You Measure Gas In Chemistry This particular gas law is. For each concentration of hydrochloric acid, plot a graph to show: Volume of gas = moles × v m. Four quantities must be known for a. The volume of a gas may be calculated from its number of moles using: Pressure is defined as the force exerted per unit area; Volume of gas (cm 3). How Do You Measure Gas In Chemistry.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii How Do You Measure Gas In Chemistry Volume of gas (cm 3) on the vertical axis. A gas syringe (left) or trough (right) should be used to measure a volume of gas. P gas = pressure of confined gas. This particular gas law is. P a = atmospheric pressure, p hg = pressure of mercury column; The liquid is placed in the u shaped tube connected to. How Do You Measure Gas In Chemistry.
From www.slideserve.com
PPT Gas Volumes L.O. PowerPoint Presentation, free download ID5877069 How Do You Measure Gas In Chemistry It can be measured using a barometer or manometer. Four quantities must be known for a. Time (s) on the horizontal axis. For each concentration of hydrochloric acid, plot a graph to show: P gas = pressure of confined gas. P a = atmospheric pressure, p hg = pressure of mercury column; The volume of a gas may be calculated. How Do You Measure Gas In Chemistry.
From chem.libretexts.org
4 The Properties of Oxygen Gas (Experiment) Chemistry LibreTexts How Do You Measure Gas In Chemistry P gas = pressure of confined gas. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. The syringe is more easily operated but has a greater. The liquid is placed in the u shaped tube connected to the big. P a = atmospheric pressure, p hg = pressure of. How Do You Measure Gas In Chemistry.
From www.youtube.com
Gas Stoichiometry Examples 1 & 2 YouTube How Do You Measure Gas In Chemistry P a = atmospheric pressure, p hg = pressure of mercury column; A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. The syringe is more easily operated but has a greater. The liquid is placed in the u shaped tube connected to the big. A gas syringe (left) or. How Do You Measure Gas In Chemistry.
From ecampusontario.pressbooks.pub
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas How Do You Measure Gas In Chemistry Pressure is defined as the force exerted per unit area; A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. Time (s) on the horizontal axis. This particular gas law is. It can be measured using a barometer or manometer. Volume of gas (cm 3) on the vertical axis. P. How Do You Measure Gas In Chemistry.
From www.azom.com
An Introduction to Gas Measurement How Do You Measure Gas In Chemistry Volume of gas = moles × v m. Volume of gas (cm 3) on the vertical axis. Time (s) on the horizontal axis. It can be measured using a barometer or manometer. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. The volume of a gas may be calculated. How Do You Measure Gas In Chemistry.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps How Do You Measure Gas In Chemistry The syringe is more easily operated but has a greater. It can be measured using a barometer or manometer. A gas syringe (left) or trough (right) should be used to measure a volume of gas. Pressure is defined as the force exerted per unit area; This particular gas law is. Four quantities must be known for a. Time (s) on. How Do You Measure Gas In Chemistry.
From atomstalk.com
The Gas Laws AtomsTalk How Do You Measure Gas In Chemistry The syringe is more easily operated but has a greater. This particular gas law is. P gas = pressure of confined gas. The liquid is placed in the u shaped tube connected to the big. It can be measured using a barometer or manometer. A gas syringe (left) or trough (right) should be used to measure a volume of gas.. How Do You Measure Gas In Chemistry.
From chem.libretexts.org
Chapter 10.6 Stoichiometry Involving Gases Chemistry LibreTexts How Do You Measure Gas In Chemistry Volume of gas = moles × v m. The liquid is placed in the u shaped tube connected to the big. The syringe is more easily operated but has a greater. Four quantities must be known for a. Pressure is defined as the force exerted per unit area; Volume of gas (cm 3) on the vertical axis. This particular gas. How Do You Measure Gas In Chemistry.
From mmerevise.co.uk
The Ideal Gas Equation MME How Do You Measure Gas In Chemistry For each concentration of hydrochloric acid, plot a graph to show: Volume of gas (cm 3) on the vertical axis. The liquid is placed in the u shaped tube connected to the big. Four quantities must be known for a. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas.. How Do You Measure Gas In Chemistry.
From sciencenotes.org
Ideal Gas Law Formula and Examples How Do You Measure Gas In Chemistry It can be measured using a barometer or manometer. Four quantities must be known for a. Time (s) on the horizontal axis. For each concentration of hydrochloric acid, plot a graph to show: The syringe is more easily operated but has a greater. The volume of a gas may be calculated from its number of moles using: Pressure is defined. How Do You Measure Gas In Chemistry.
From www.chegg.com
Solved Sometimes in lab we collect the gas formed by a How Do You Measure Gas In Chemistry Four quantities must be known for a. It can be measured using a barometer or manometer. A gas syringe (left) or trough (right) should be used to measure a volume of gas. P a = atmospheric pressure, p hg = pressure of mercury column; For each concentration of hydrochloric acid, plot a graph to show: Volume of gas = moles. How Do You Measure Gas In Chemistry.
From www.slideserve.com
PPT Gas Density PowerPoint Presentation, free download ID3286907 How Do You Measure Gas In Chemistry Four quantities must be known for a. Volume of gas = moles × v m. The liquid is placed in the u shaped tube connected to the big. It can be measured using a barometer or manometer. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. Time (s) on. How Do You Measure Gas In Chemistry.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial How Do You Measure Gas In Chemistry The volume of a gas may be calculated from its number of moles using: Time (s) on the horizontal axis. Pressure is defined as the force exerted per unit area; A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. A gas syringe (left) or trough (right) should be used. How Do You Measure Gas In Chemistry.
From videos.mathtutordvd.com
Chemistry 1 Vol 5 Gas Laws & Theory Math Tutor Public Gallery How Do You Measure Gas In Chemistry Volume of gas = moles × v m. For each concentration of hydrochloric acid, plot a graph to show: Pressure is defined as the force exerted per unit area; The syringe is more easily operated but has a greater. Four quantities must be known for a. It can be measured using a barometer or manometer. A gas law is a. How Do You Measure Gas In Chemistry.
From www.wikihow.com
3 Ways to Measure Density of Gases wikiHow How Do You Measure Gas In Chemistry The volume of a gas may be calculated from its number of moles using: Time (s) on the horizontal axis. Volume of gas = moles × v m. For each concentration of hydrochloric acid, plot a graph to show: P gas = pressure of confined gas. This particular gas law is. Four quantities must be known for a. Volume of. How Do You Measure Gas In Chemistry.
From www.slideserve.com
PPT Gas Volumes L.O. PowerPoint Presentation, free download ID5877069 How Do You Measure Gas In Chemistry A gas syringe (left) or trough (right) should be used to measure a volume of gas. It can be measured using a barometer or manometer. Time (s) on the horizontal axis. P a = atmospheric pressure, p hg = pressure of mercury column; Four quantities must be known for a. For each concentration of hydrochloric acid, plot a graph to. How Do You Measure Gas In Chemistry.
From courses.lumenlearning.com
Stoichiometry of Gaseous Substances, Mixtures, and Reactions How Do You Measure Gas In Chemistry The syringe is more easily operated but has a greater. Volume of gas (cm 3) on the vertical axis. Volume of gas = moles × v m. Time (s) on the horizontal axis. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. It can be measured using a barometer. How Do You Measure Gas In Chemistry.
From en.ppt-online.org
The ideal gas equation online presentation How Do You Measure Gas In Chemistry The volume of a gas may be calculated from its number of moles using: The liquid is placed in the u shaped tube connected to the big. Four quantities must be known for a. It can be measured using a barometer or manometer. Time (s) on the horizontal axis. For each concentration of hydrochloric acid, plot a graph to show:. How Do You Measure Gas In Chemistry.
From www.slideserve.com
PPT Units of Measure for Gases PowerPoint Presentation, free download How Do You Measure Gas In Chemistry For each concentration of hydrochloric acid, plot a graph to show: The volume of a gas may be calculated from its number of moles using: It can be measured using a barometer or manometer. This particular gas law is. Time (s) on the horizontal axis. The liquid is placed in the u shaped tube connected to the big. A gas. How Do You Measure Gas In Chemistry.