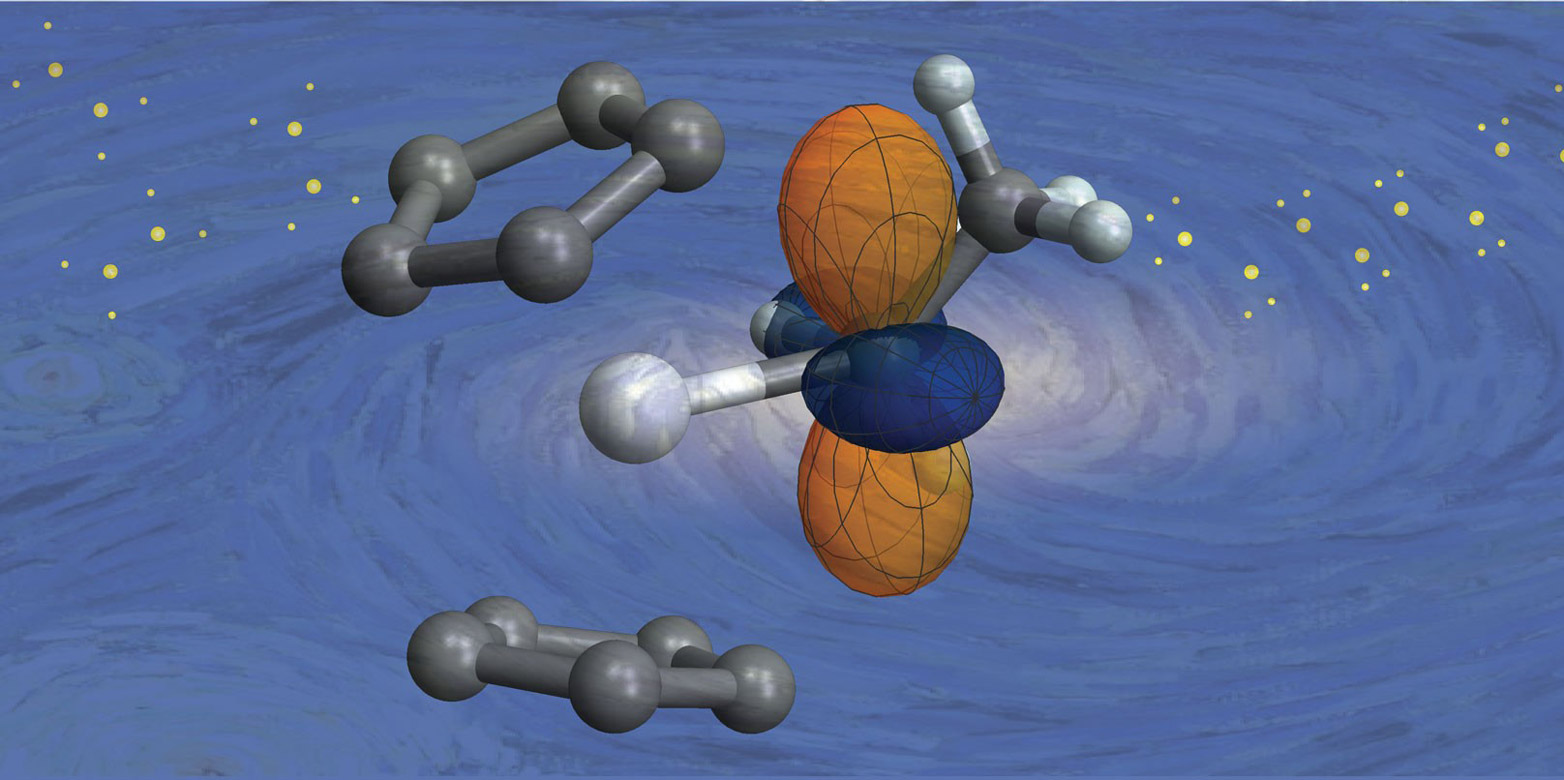
Catalysts Chemical Reactivity . Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Therefore they are crucially important in chemical industry, exhaust gas treatment and other chemical reactions. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. This process is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Each catalyst molecule may induce the transformation of many molecules of reactants. While the chemical essence of catalysis is obscure, practical catalysts have been developed based on the accumulation of empirical. Another reason for using a catalyst is that. It is not consumed by the process. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Learn about the history, classification, and reactions of catalysis. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. A catalyst lowers the activation energy of a reaction, increasing its rate.
from ethz.ch
In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. This process is called catalysis. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Therefore they are crucially important in chemical industry, exhaust gas treatment and other chemical reactions. Learn about the history, classification, and reactions of catalysis. Another reason for using a catalyst is that. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is the process of speeding up a reaction using a A catalyst lowers the activation energy of a reaction, increasing its rate.
Tracking down the reactivity of catalysts ETH Zurich
Catalysts Chemical Reactivity A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Each catalyst molecule may induce the transformation of many molecules of reactants. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. Catalysis is the process of speeding up a reaction using a Therefore they are crucially important in chemical industry, exhaust gas treatment and other chemical reactions. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. While the chemical essence of catalysis is obscure, practical catalysts have been developed based on the accumulation of empirical. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Learn about the history, classification, and reactions of catalysis. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. In general, catalytic action is a chemical reaction between the catalyst and a reactant. This process is called catalysis. A catalyst lowers the activation energy of a reaction, increasing its rate.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2684800 Catalysts Chemical Reactivity Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Therefore they are crucially important in chemical industry, exhaust gas treatment and other chemical reactions. Learn about the history, classification, and reactions of catalysis. A catalyst lowers the activation energy of a reaction, increasing its rate. A catalyst is another substance than reactants products. Catalysts Chemical Reactivity.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalysts Chemical Reactivity It is not consumed by the process. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Catalysis is the process of. Catalysts Chemical Reactivity.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalysts Chemical Reactivity This process is called catalysis. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst lowers the activation energy of a reaction, increasing its rate. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. While the. Catalysts Chemical Reactivity.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalysts Chemical Reactivity A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysis is the process of speeding up a reaction using a Learn about the history, classification, and reactions of catalysis. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Therefore they are crucially important in chemical industry,. Catalysts Chemical Reactivity.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysts Chemical Reactivity While the chemical essence of catalysis is obscure, practical catalysts have been developed based on the accumulation of empirical. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Another reason for using a catalyst is that. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Therefore. Catalysts Chemical Reactivity.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalysts Chemical Reactivity Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Each catalyst molecule may induce the transformation of many molecules of reactants. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction.. Catalysts Chemical Reactivity.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalysts Chemical Reactivity In general, catalytic action is a chemical reaction between the catalyst and a reactant. While the chemical essence of catalysis is obscure, practical catalysts have been developed based on the accumulation of empirical. Catalysis is the process of speeding up a reaction using a A catalyst is another substance than reactants products added to a reaction system to alter the. Catalysts Chemical Reactivity.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalysts Chemical Reactivity A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. While the chemical essence of catalysis is obscure, practical catalysts have been. Catalysts Chemical Reactivity.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalysts Chemical Reactivity This process is called catalysis. A catalyst lowers the activation energy of a reaction, increasing its rate. Therefore they are crucially important in chemical industry, exhaust gas treatment and other chemical reactions. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is. Catalysts Chemical Reactivity.
From www.youtube.com
6.2.6 / 6.2.7 Describe the effect of a catalyst on a chemical reaction Catalysts Chemical Reactivity In general, catalytic action is a chemical reaction between the catalyst and a reactant. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Each catalyst molecule may induce the transformation of many molecules of reactants. While the chemical essence of catalysis is obscure, practical. Catalysts Chemical Reactivity.
From chemwiki.ucdavis.edu
Catalytic Hydrogenation of Alkenes Chemwiki Catalysts Chemical Reactivity A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction.. Catalysts Chemical Reactivity.
From ethz.ch
Tracking down the reactivity of catalysts ETH Zurich Catalysts Chemical Reactivity Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst lowers the activation energy of a reaction, increasing its rate. This process is called catalysis. Each catalyst molecule may induce the transformation of many molecules of reactants. It is not consumed by the process. Another reason for using a catalyst is that.. Catalysts Chemical Reactivity.
From wechemistry.blogspot.com
Why Do Chemicals React? and Thermodynamics Chemistry Solutions Catalysts Chemical Reactivity It is not consumed by the process. Therefore they are crucially important in chemical industry, exhaust gas treatment and other chemical reactions. Learn about the history, classification, and reactions of catalysis. Catalysis is the process of speeding up a reaction using a Each catalyst molecule may induce the transformation of many molecules of reactants. It interacts with the reactants in. Catalysts Chemical Reactivity.
From www.msjchem.com
Reactivity 2.2 How fast? The rate of chemical change MSJChem Catalysts Chemical Reactivity While the chemical essence of catalysis is obscure, practical catalysts have been developed based on the accumulation of empirical. Catalysis is the process of speeding up a reaction using a In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Each catalyst molecule may induce the transformation of. Catalysts Chemical Reactivity.
From 2012books.lardbucket.org
Catalysis Catalysts Chemical Reactivity It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. While the chemical essence of catalysis is obscure, practical catalysts have been developed based on the accumulation of empirical. Therefore they are crucially important in chemical industry, exhaust gas treatment and other chemical reactions. In chemistry. Catalysts Chemical Reactivity.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalysts Chemical Reactivity While the chemical essence of catalysis is obscure, practical catalysts have been developed based on the accumulation of empirical. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. This process is called catalysis. Learn about the history, classification, and reactions of catalysis. Catalysts reduce the activation energy of reactions and enhance. Catalysts Chemical Reactivity.
From www.pinterest.co.uk
Catalysis Chemistry classroom, Chemistry Catalysts Chemical Reactivity Learn about the history, classification, and reactions of catalysis. Each catalyst molecule may induce the transformation of many molecules of reactants. Catalysis is the process of speeding up a reaction using a Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is not consumed by the reaction and it may participate. Catalysts Chemical Reactivity.
From www.chemistrylearner.com
Reactivity Series Definition and Chart Catalysts Chemical Reactivity Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. It is not consumed by the process. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst lowers the activation energy of a reaction, increasing its rate.. Catalysts Chemical Reactivity.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalysts Chemical Reactivity Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst lowers the activation energy of a reaction, increasing its rate. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. While the chemical essence of catalysis is. Catalysts Chemical Reactivity.
From www.tes.com
Secondary chemistry teaching resources Chemical reactions TES Catalysts Chemical Reactivity Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Each catalyst molecule may induce the transformation of many molecules of reactants. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. In chemistry and biology, a catalyst is a substance the increases the. Catalysts Chemical Reactivity.
From www.youtube.com
6.1 Describe the effect of a catalyst on a chemical reaction [SL IB Catalysts Chemical Reactivity In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. This process is called catalysis. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a. Catalysts Chemical Reactivity.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalysts Chemical Reactivity A catalyst lowers the activation energy of a reaction, increasing its rate. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. It is not consumed by the process. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a. Catalysts Chemical Reactivity.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalysts Chemical Reactivity A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysis, the modification of the rate of a chemical reaction,. Catalysts Chemical Reactivity.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalysts Chemical Reactivity Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Catalysis is the process of speeding up a reaction using a It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. Learn about the history, classification, and reactions of catalysis. While the. Catalysts Chemical Reactivity.
From www.tes.com
The Effect of Catalysts Teaching Resources Catalysts Chemical Reactivity Therefore they are crucially important in chemical industry, exhaust gas treatment and other chemical reactions. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Catalysis is the process of speeding up a reaction using a Learn about the history, classification, and reactions of catalysis. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions.. Catalysts Chemical Reactivity.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalysts Chemical Reactivity It is not consumed by the process. While the chemical essence of catalysis is obscure, practical catalysts have been developed based on the accumulation of empirical. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. A catalyst is not consumed by the reaction and it may. Catalysts Chemical Reactivity.
From www.wisegeek.org
What is a Catalyst? (with pictures) Catalysts Chemical Reactivity Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Another reason for using a catalyst is that. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions.. Catalysts Chemical Reactivity.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Catalysts Chemical Reactivity In general, catalytic action is a chemical reaction between the catalyst and a reactant. This process is called catalysis. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. A catalyst is another substance than reactants products added to a reaction system to alter the speed. Catalysts Chemical Reactivity.
From www.waca.msf.org
Lesson Explainer Catalysts, Catalyst Catalysts Chemical Reactivity Learn about the history, classification, and reactions of catalysis. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. In chemistry. Catalysts Chemical Reactivity.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalysts Chemical Reactivity In general, catalytic action is a chemical reaction between the catalyst and a reactant. Another reason for using a catalyst is that. Therefore they are crucially important in chemical industry, exhaust gas treatment and other chemical reactions. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysis is the process of speeding up a reaction using a. Catalysts Chemical Reactivity.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalysts Chemical Reactivity While the chemical essence of catalysis is obscure, practical catalysts have been developed based on the accumulation of empirical. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. Therefore they are crucially important in chemical industry, exhaust gas treatment and other chemical reactions. This process. Catalysts Chemical Reactivity.
From chem.unc.edu
Molecular Catalysts Department of Chemistry Catalysts Chemical Reactivity Catalysis is the process of speeding up a reaction using a A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Each catalyst molecule may induce the. Catalysts Chemical Reactivity.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Chemical Reactivity Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn about the history, classification, and reactions of catalysis. This process is called catalysis. Therefore they are crucially important. Catalysts Chemical Reactivity.
From getrevising.co.uk
The Reactivity Series Revision Notes in GCSE Chemistry Catalysts Chemical Reactivity Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Another reason for using a catalyst is that. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Learn about the history, classification, and reactions of catalysis. A catalyst is a chemical substance that affects the rate of a chemical. Catalysts Chemical Reactivity.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalysts Chemical Reactivity It is not consumed by the process. Another reason for using a catalyst is that. Catalysis is the process of speeding up a reaction using a A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Enzymes are naturally occurring catalysts responsible for many essential. Catalysts Chemical Reactivity.