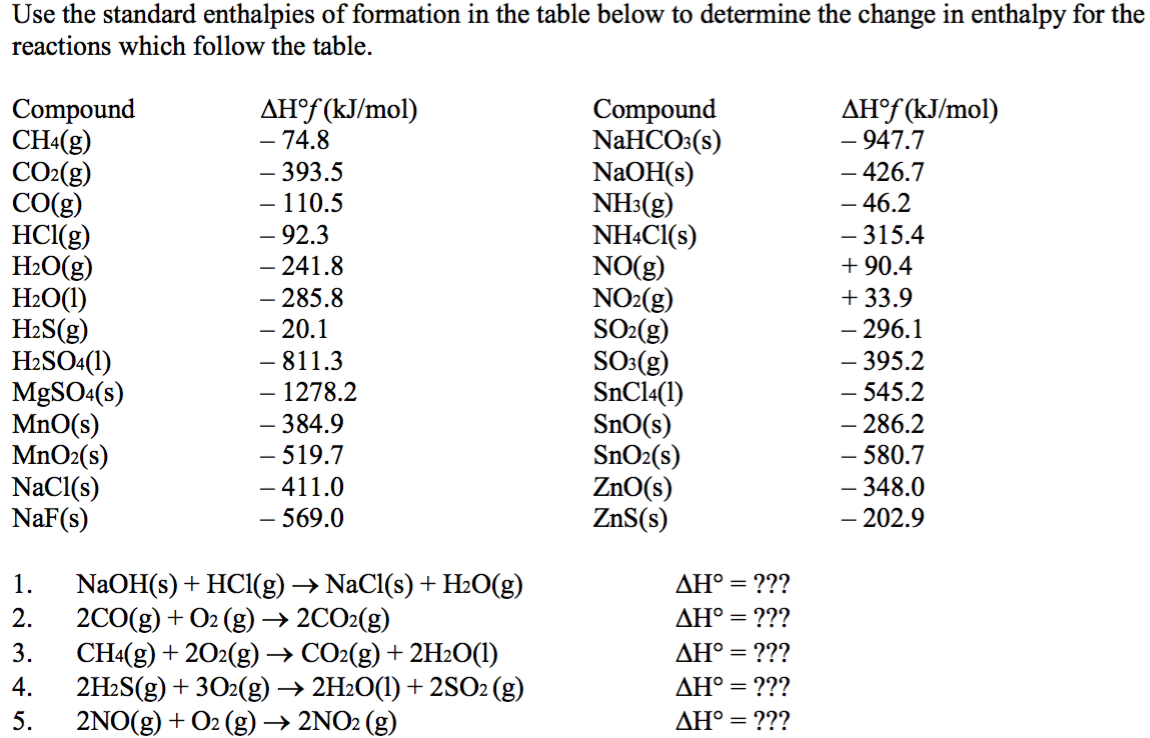
Standard Enthalpy Of Formation Worksheet . 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. The standard enthalpy and entropy of various. Calculate δh° rxn for this reaction using standard enthalpies of formation. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. Calculate the enthalpy of this reaction using standard enthalpies of formation. Calculate h o in kilojoules for the following reactions. Standard heats of formation worksheet 1. If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change.
from www.chegg.com
If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy and entropy of various. Calculate the enthalpy of this reaction using standard enthalpies of formation. Standard heats of formation worksheet 1. Calculate h o in kilojoules for the following reactions. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. Calculate δh° rxn for this reaction using standard enthalpies of formation. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its.
Solved Use the standard enthalpies of formation in the table
Standard Enthalpy Of Formation Worksheet If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change. If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. Calculate h o in kilojoules for the following reactions. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. The standard enthalpy and entropy of various. Calculate the enthalpy of this reaction using standard enthalpies of formation. Calculate δh° rxn for this reaction using standard enthalpies of formation. Standard heats of formation worksheet 1.
From www.chegg.com
Heat of Formation Worksheet Use a standard enthalpies Standard Enthalpy Of Formation Worksheet Calculate δh° rxn for this reaction using standard enthalpies of formation. The standard enthalpy and entropy of various. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. If a compound is. Standard Enthalpy Of Formation Worksheet.
From lessonmagicprotasis.z13.web.core.windows.net
Enthalpy Worksheets With Answers Standard Enthalpy Of Formation Worksheet Calculate δh° rxn for this reaction using standard enthalpies of formation. Standard heats of formation worksheet 1. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change. 4) standard enthalpy change of formation. Standard Enthalpy Of Formation Worksheet.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation Worksheet Calculate δh° rxn for this reaction using standard enthalpies of formation. Calculate the enthalpy of this reaction using standard enthalpies of formation. Standard heats of formation worksheet 1. If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change. Calculate h o in kilojoules for the following reactions.. Standard Enthalpy Of Formation Worksheet.
From worksheets.ekocraft-appleleaf.com
Enthalpy Worksheet Worksheets For Kindergarten Standard Enthalpy Of Formation Worksheet Calculate the enthalpy of this reaction using standard enthalpies of formation. The standard enthalpy and entropy of various. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. Calculate δh° rxn for this reaction using standard enthalpies of formation. The standard enthalpy of formation is a measure of. Standard Enthalpy Of Formation Worksheet.
From www.coursehero.com
[Solved] Unit 3 SCH4U Worksheet Dr. sarshar Enthalpy of Formation Standard Enthalpy Of Formation Worksheet Calculate δh° rxn for this reaction using standard enthalpies of formation. Standard heats of formation worksheet 1. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. The standard enthalpy and entropy of various. If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy. Standard Enthalpy Of Formation Worksheet.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Worksheet Calculate δh° rxn for this reaction using standard enthalpies of formation. Standard heats of formation worksheet 1. Calculate h o in kilojoules for the following reactions. The standard enthalpy and entropy of various. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. If a compound. Standard Enthalpy Of Formation Worksheet.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Worksheet Standard heats of formation worksheet 1. Calculate δh° rxn for this reaction using standard enthalpies of formation. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. The standard enthalpy and entropy of various. If a compound is formed from the elements it contains in their naturally occurring. Standard Enthalpy Of Formation Worksheet.
From www.formsbank.com
Enthalpy Worksheet With Answers printable pdf download Standard Enthalpy Of Formation Worksheet Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change. The. Standard Enthalpy Of Formation Worksheet.
From learningschoolmorticed.z14.web.core.windows.net
Enthalpy Of Formation Worksheet With Answers Standard Enthalpy Of Formation Worksheet The standard enthalpy and entropy of various. Calculate h o in kilojoules for the following reactions. Standard heats of formation worksheet 1. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Enthalpy Of Formation Worksheet.
From ataglance.randstad.com
Enthalpy Of Formation Worksheet Printable Calendars AT A GLANCE Standard Enthalpy Of Formation Worksheet If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change. The standard enthalpy and entropy of various. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of. Standard Enthalpy Of Formation Worksheet.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Worksheet The standard enthalpy and entropy of various. Standard heats of formation worksheet 1. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. If a compound is formed from the elements it contains in. Standard Enthalpy Of Formation Worksheet.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Worksheet 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Calculate h o in kilojoules for the following reactions. Calculate δh° rxn for this. Standard Enthalpy Of Formation Worksheet.
From studylib.net
enthalpy worksheet Standard Enthalpy Of Formation Worksheet If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change. The standard enthalpy and entropy of various. Standard heats of formation worksheet 1. Calculate the enthalpy of this reaction using standard enthalpies of formation. Enthalpy wórksheet use the following heat of formation table in questions 2 —. Standard Enthalpy Of Formation Worksheet.
From worksheets.clipart-library.com
Free enthalpy worksheet, Download Free enthalpy worksheet png images Standard Enthalpy Of Formation Worksheet If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change. The standard enthalpy and entropy of various. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Calculate the enthalpy of this reaction. Standard Enthalpy Of Formation Worksheet.
From www.studocu.com
Chem 1 Chemistry HW Heat of Formation Worksheet Use a standard Standard Enthalpy Of Formation Worksheet The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Calculate the enthalpy of this reaction using standard enthalpies of formation. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. Calculate δh° rxn. Standard Enthalpy Of Formation Worksheet.
From lessonmagictirolese.z14.web.core.windows.net
Enthalpy Of Formation Worksheet Standard Enthalpy Of Formation Worksheet Standard heats of formation worksheet 1. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. Calculate the enthalpy of this reaction using standard enthalpies of formation. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. The standard enthalpy of formation is a. Standard Enthalpy Of Formation Worksheet.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Worksheet The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. If a compound is formed from the elements it contains in their naturally occurring. Standard Enthalpy Of Formation Worksheet.
From worksheets.clipart-library.com
Solved Enthalpy of Formation Worksheet Use standard Standard Enthalpy Of Formation Worksheet Calculate the enthalpy of this reaction using standard enthalpies of formation. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. Standard heats of formation worksheet 1. If a compound is formed from the. Standard Enthalpy Of Formation Worksheet.
From www.chegg.com
Solved Use a standard enthalpies of formation table to Standard Enthalpy Of Formation Worksheet The standard enthalpy and entropy of various. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Calculate the enthalpy of this reaction using. Standard Enthalpy Of Formation Worksheet.
From www.madebyteachers.com
Standard Molar Enthalpy of Formation Calculations A Chemistry Standard Enthalpy Of Formation Worksheet The standard enthalpy and entropy of various. Calculate h o in kilojoules for the following reactions. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. Standard heats of formation worksheet 1. Calculate the enthalpy of this reaction using standard enthalpies of formation. The standard enthalpy of formation. Standard Enthalpy Of Formation Worksheet.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Worksheet The standard enthalpy and entropy of various. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Enthalpy Of Formation Worksheet.
From study.com
Quiz & Worksheet Standard Enthalpy of Formation Standard Enthalpy Of Formation Worksheet The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Calculate h o in kilojoules for the following reactions. The standard enthalpy and entropy of various. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed. Standard Enthalpy Of Formation Worksheet.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Worksheet If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change. Calculate h o in kilojoules for the following reactions. Calculate δh° rxn for this reaction using standard enthalpies of formation. The standard enthalpy and entropy of various. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”). Standard Enthalpy Of Formation Worksheet.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Worksheet Calculate the enthalpy of this reaction using standard enthalpies of formation. Calculate δh° rxn for this reaction using standard enthalpies of formation. Standard heats of formation worksheet 1. The standard enthalpy and entropy of various. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Enthalpy. Standard Enthalpy Of Formation Worksheet.
From www.chegg.com
Solved Enthalpy of Formation Worksheet Use standard Standard Enthalpy Of Formation Worksheet Standard heats of formation worksheet 1. Calculate δh° rxn for this reaction using standard enthalpies of formation. Calculate the enthalpy of this reaction using standard enthalpies of formation. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Enthalpy wórksheet use the following heat of formation. Standard Enthalpy Of Formation Worksheet.
From thekidsworksheet.com
Thermochemistry Standard Heats Of Formation Worksheet Thekidsworksheet Standard Enthalpy Of Formation Worksheet Standard heats of formation worksheet 1. The standard enthalpy and entropy of various. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. Calculate. Standard Enthalpy Of Formation Worksheet.
From printablelibvera.z13.web.core.windows.net
Enthalpy Of Formation Worksheet Standard Enthalpy Of Formation Worksheet The standard enthalpy and entropy of various. Calculate δh° rxn for this reaction using standard enthalpies of formation. Standard heats of formation worksheet 1. Calculate h o in kilojoules for the following reactions. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. Calculate the enthalpy of this reaction using standard enthalpies of formation. The standard. Standard Enthalpy Of Formation Worksheet.
From studylib.net
Enthalpy worksheets 1& 2 Standard Enthalpy Of Formation Worksheet The standard enthalpy and entropy of various. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. Calculate δh° rxn for this reaction using standard enthalpies of formation. Calculate h o in kilojoules for the following reactions. Standard heats of formation worksheet 1. Calculate the enthalpy of this reaction using standard enthalpies of formation. The standard. Standard Enthalpy Of Formation Worksheet.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy Standard Enthalpy Of Formation Worksheet The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. If a compound is formed from the elements it contains in their naturally occurring forms, the enthalpy change is called the enthalpy change. Enthalpy wórksheet use the following heat of formation table in questions 2 —. Standard Enthalpy Of Formation Worksheet.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Worksheet 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. Calculate δh° rxn for this reaction using standard enthalpies of formation. Calculate the enthalpy of this reaction using standard enthalpies of formation. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Enthalpy Of Formation Worksheet.
From www.chegg.com
Solved Heat of Formation Worksheet Use a standard enthalpies Standard Enthalpy Of Formation Worksheet 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. Calculate the enthalpy of this reaction using standard enthalpies of formation. The standard enthalpy and entropy of various. Calculate δh° rxn for this reaction using standard enthalpies of formation. The standard enthalpy of formation is a measure of. Standard Enthalpy Of Formation Worksheet.
From www.chegg.com
Solved Standard Enthalpy of Formation Worksheet Use the Standard Enthalpy Of Formation Worksheet Calculate h o in kilojoules for the following reactions. Standard heats of formation worksheet 1. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Calculate the enthalpy of this reaction using standard enthalpies of formation. Calculate δh° rxn for this reaction using standard enthalpies of. Standard Enthalpy Of Formation Worksheet.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Worksheet Calculate δh° rxn for this reaction using standard enthalpies of formation. Calculate the enthalpy of this reaction using standard enthalpies of formation. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Enthalpy Of Formation Worksheet.
From gmbar.co
️Standard Enthalpy Of Formation Worksheet Free Download Gmbar.co Standard Enthalpy Of Formation Worksheet Calculate δh° rxn for this reaction using standard enthalpies of formation. Standard heats of formation worksheet 1. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its. Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. Calculate h o in kilojoules for the. Standard Enthalpy Of Formation Worksheet.
From www.madebyteachers.com
Standard Molar Enthalpy of Formation Calculations A Chemistry Standard Enthalpy Of Formation Worksheet Enthalpy wórksheet use the following heat of formation table in questions 2 — 6. Calculate the enthalpy of this reaction using standard enthalpies of formation. The standard enthalpy and entropy of various. Standard heats of formation worksheet 1. 4) standard enthalpy change of formation (dfh°) (“enthalpy of formation”) enthalpy change when 1 mole of a substance is formed from its.. Standard Enthalpy Of Formation Worksheet.