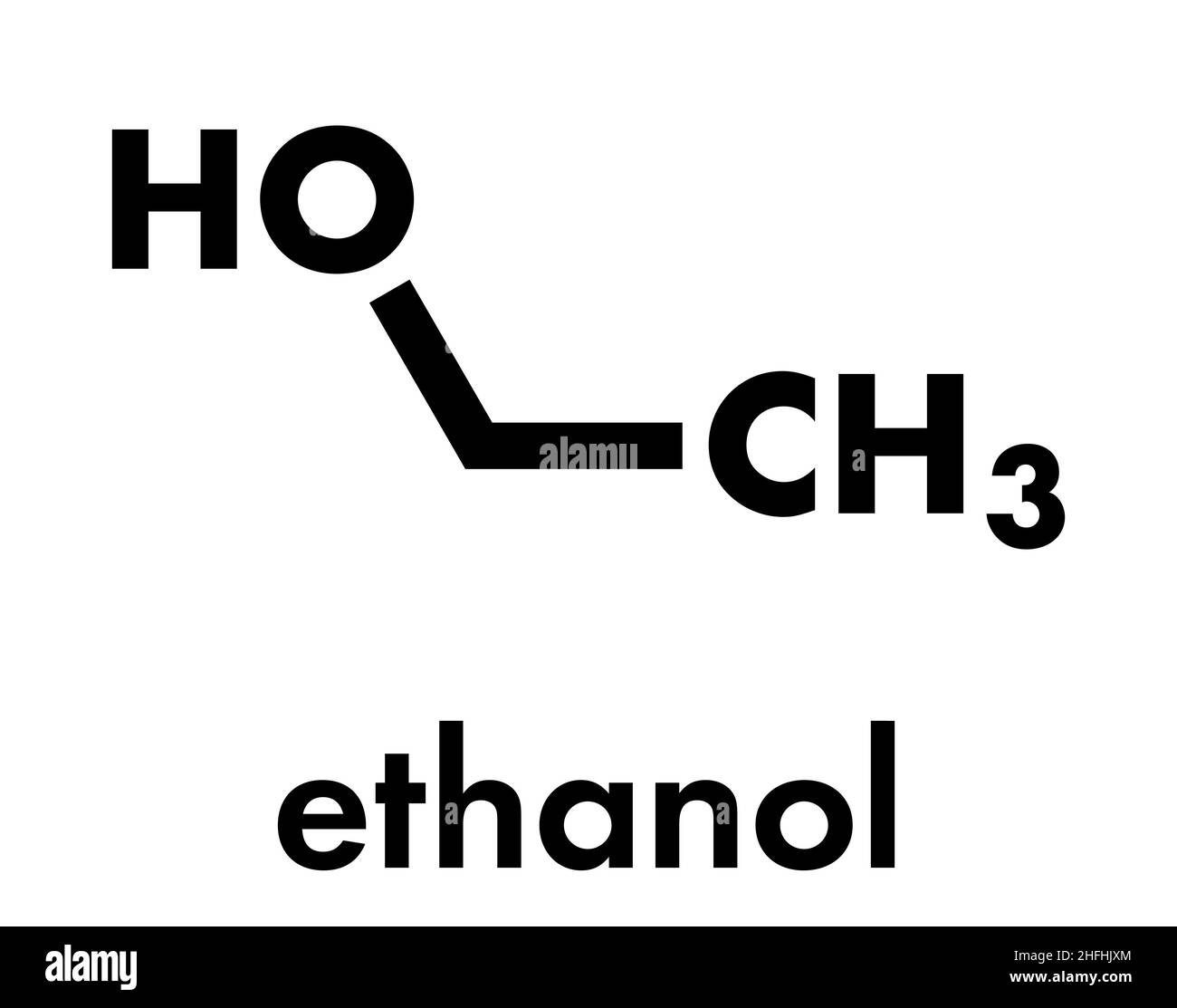
Why Is Ethanol In Alcohol . In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Ethanol, or ethyl alcohol, is the only type of alcohol. In both pure water and pure ethanol the main intermolecular attractions are hydrogen. consider ethanol as a typical small alcohol. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. alcohols, like water, are both weak bases and weak acids. the distinction between alcohol and ethanol is pretty simple: There are two main processes for.
from www.alamy.com
consider ethanol as a typical small alcohol. There are two main processes for. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. alcohols, like water, are both weak bases and weak acids. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. the distinction between alcohol and ethanol is pretty simple: simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Ethanol, or ethyl alcohol, is the only type of alcohol. In both pure water and pure ethanol the main intermolecular attractions are hydrogen.
Ethanol fuel ethanol ethyl alcohol Black and White Stock Photos
Why Is Ethanol In Alcohol alcohols, like water, are both weak bases and weak acids. consider ethanol as a typical small alcohol. There are two main processes for. Ethanol, or ethyl alcohol, is the only type of alcohol. In both pure water and pure ethanol the main intermolecular attractions are hydrogen. simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. the distinction between alcohol and ethanol is pretty simple: In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. alcohols, like water, are both weak bases and weak acids. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits.
From www.wisegeek.com
What is Ethanol Alcohol? (with pictures) Why Is Ethanol In Alcohol the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. In aqueous. Why Is Ethanol In Alcohol.
From www.dreamstime.com
Ethanol, Ethyl Alcohol Molecule, Chemical Structure. Skeletal Formula Why Is Ethanol In Alcohol There are two main processes for. simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. consider ethanol as a typical small alcohol. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. Ethanol, or ethyl alcohol, is the only type of. Why Is Ethanol In Alcohol.
From www.youtube.com
Alcohol Poisoning Ethanol and Methanol YouTube Why Is Ethanol In Alcohol consider ethanol as a typical small alcohol. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. There are two main processes for. alcohols, like water, are both weak bases and weak acids. simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. the 'alcohol' in. Why Is Ethanol In Alcohol.
From www.pdfprof.com
alcohol metabolism chart Why Is Ethanol In Alcohol the distinction between alcohol and ethanol is pretty simple: There are two main processes for. alcohols, like water, are both weak bases and weak acids. In both pure water and pure ethanol the main intermolecular attractions are hydrogen. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and. Why Is Ethanol In Alcohol.
From www.alamy.com
Ethanol fuel ethanol ethyl alcohol Black and White Stock Photos Why Is Ethanol In Alcohol the distinction between alcohol and ethanol is pretty simple: ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Ethanol, or ethyl alcohol, is the only type of alcohol. In both pure water and pure ethanol the main intermolecular. Why Is Ethanol In Alcohol.
From www.alamy.com
Ethanol Alcohol Molecular structure vector skeletal formula Stock Why Is Ethanol In Alcohol the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. In both pure water and pure ethanol the main intermolecular attractions are hydrogen. consider ethanol as a typical small alcohol. simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized. Why Is Ethanol In Alcohol.
From www.chemicals.co.uk
What is Ethyl Alcohol? Why Is Ethanol In Alcohol There are two main processes for. Ethanol, or ethyl alcohol, is the only type of alcohol. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. alcohols, like water, are both weak bases and weak acids. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and. Why Is Ethanol In Alcohol.
From www.reddit.com
New and old ethanol bottles. Looks like ethanol has worse for Why Is Ethanol In Alcohol In both pure water and pure ethanol the main intermolecular attractions are hydrogen. simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. Ethanol, or ethyl alcohol, is the only type of alcohol. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits.. Why Is Ethanol In Alcohol.
From cartoondealer.com
Ethanol, Pure Ethyl Alcohol In Bottle RoyaltyFree Stock Photography Why Is Ethanol In Alcohol There are two main processes for. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. alcohols, like water, are both weak bases and weak acids. the distinction between alcohol and ethanol is pretty simple: consider ethanol as a typical small alcohol. ethanol is also the intoxicating ingredient of many alcoholic beverages such as. Why Is Ethanol In Alcohol.
From www.lecturio.com
Ethanol Metabolism Concise Medical Knowledge Why Is Ethanol In Alcohol There are two main processes for. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. the distinction between alcohol and ethanol is pretty simple: In both pure water and pure ethanol the main intermolecular attractions. Why Is Ethanol In Alcohol.
From www.activeelement.org
ethanol 40B Archives Active Element Why Is Ethanol In Alcohol the distinction between alcohol and ethanol is pretty simple: alcohols, like water, are both weak bases and weak acids. Ethanol, or ethyl alcohol, is the only type of alcohol. In both pure water and pure ethanol the main intermolecular attractions are hydrogen. There are two main processes for. the 'alcohol' in alcoholic drinks is ethanol, produced by. Why Is Ethanol In Alcohol.
From chemicaldb.netlify.app
Why is ethanoic acid considered an acid Why Is Ethanol In Alcohol the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. alcohols, like water, are both weak bases and weak acids. Ethanol, or ethyl alcohol, is the only type of alcohol. simple alcohols like methanol, ethanol,. Why Is Ethanol In Alcohol.
From www.eromman.com
Buy Ethanol 95 Sanitizer Food Grade Undenatured Ethyl Alcohol 500ml Why Is Ethanol In Alcohol ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. In both pure water and pure ethanol the main intermolecular attractions are hydrogen. There are two main processes for. consider ethanol as a typical small alcohol. simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and. Why Is Ethanol In Alcohol.
From a-z-animals.com
Discover the Molar Mass of Ethyl Alcohol (Ethanol) + Key Examples of Why Is Ethanol In Alcohol In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. There are two main processes for. simple alcohols. Why Is Ethanol In Alcohol.
From www.youtube.com
Boiling Point for C2H5OH (Ethanol or Ethyl Alcohol) YouTube Why Is Ethanol In Alcohol Ethanol, or ethyl alcohol, is the only type of alcohol. consider ethanol as a typical small alcohol. the distinction between alcohol and ethanol is pretty simple: simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. In. Why Is Ethanol In Alcohol.
From www.teachoo.com
[Carbon Class 10] Ethanol Physical and Chemical Properties, Uses Why Is Ethanol In Alcohol ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. Ethanol, or ethyl alcohol, is the only type of alcohol. simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. alcohols, like water, are both weak bases and weak acids. There are. Why Is Ethanol In Alcohol.
From www.alamy.com
Alcohol (ethanol, ethyl alcohol) molecule, chemical structure. Skeletal Why Is Ethanol In Alcohol simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. the distinction between alcohol and ethanol is pretty simple: consider ethanol as a typical small alcohol. Ethanol, or. Why Is Ethanol In Alcohol.
From www.teachoo.com
[Chemistry] Differentiate between Ethanol and Ethanoic acid Class 10 Why Is Ethanol In Alcohol the distinction between alcohol and ethanol is pretty simple: simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. consider ethanol as a typical small alcohol. There are two main processes for. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Ethanol, or ethyl alcohol, is. Why Is Ethanol In Alcohol.
From www.alamy.com
Ethanol (EtOH, ethyl alcohol) molecule, chemical structure isolated Why Is Ethanol In Alcohol consider ethanol as a typical small alcohol. In both pure water and pure ethanol the main intermolecular attractions are hydrogen. simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. There are two main processes for. the distinction between alcohol and ethanol is pretty simple: In aqueous solutions, alcohols. Why Is Ethanol In Alcohol.
From www.youtube.com
Chemical and Structural Formula for Ethanol (Ethyl alcohol) YouTube Why Is Ethanol In Alcohol ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. simple alcohols like methanol, ethanol, and propanol occur. Why Is Ethanol In Alcohol.
From de.vecteezy.com
EthanolAlkoholMolekül 8530754 PNG Why Is Ethanol In Alcohol the distinction between alcohol and ethanol is pretty simple: ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. There are two main processes for. the 'alcohol' in alcoholic drinks is. Why Is Ethanol In Alcohol.
From www.alamy.com
Alcohol (ethanol, ethyl alcohol) molecule, chemical structure Stock Why Is Ethanol In Alcohol In both pure water and pure ethanol the main intermolecular attractions are hydrogen. There are two main processes for. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. the. Why Is Ethanol In Alcohol.
From letstalkscience.ca
How is Ethanol Made? Let's Talk Science Why Is Ethanol In Alcohol In both pure water and pure ethanol the main intermolecular attractions are hydrogen. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. alcohols, like water, are both weak bases and weak acids. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. the distinction between alcohol and. Why Is Ethanol In Alcohol.
From www.youtube.com
Identification test for Alcohol ethanol and methanol chemical test Why Is Ethanol In Alcohol In both pure water and pure ethanol the main intermolecular attractions are hydrogen. the distinction between alcohol and ethanol is pretty simple: There are two main processes for. consider ethanol as a typical small alcohol. alcohols, like water, are both weak bases and weak acids. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation. Why Is Ethanol In Alcohol.
From stock.adobe.com
Alcohol (ethanol, ethyl alcohol) molecule, chemical structure. Skeletal Why Is Ethanol In Alcohol the distinction between alcohol and ethanol is pretty simple: In both pure water and pure ethanol the main intermolecular attractions are hydrogen. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. alcohols, like water, are both weak bases and weak acids. Ethanol, or ethyl alcohol, is the only type. Why Is Ethanol In Alcohol.
From www.alphamedicalsolutions.com.au
Ethanol Alcohol Why Is Ethanol In Alcohol In both pure water and pure ethanol the main intermolecular attractions are hydrogen. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. There are two main processes for. alcohols, like water, are both weak bases and weak acids. simple alcohols like methanol, ethanol, and propanol occur in modest quantities. Why Is Ethanol In Alcohol.
From vitalbypoet.com
Infographics The Ethanol Process Vital A news & media resource Why Is Ethanol In Alcohol Ethanol, or ethyl alcohol, is the only type of alcohol. the distinction between alcohol and ethanol is pretty simple: ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. There. Why Is Ethanol In Alcohol.
From www.adda247.com
Ethanol Formula Ethyl Alcohol Formula, Structure Why Is Ethanol In Alcohol In both pure water and pure ethanol the main intermolecular attractions are hydrogen. alcohols, like water, are both weak bases and weak acids. consider ethanol as a typical small alcohol. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. the 'alcohol' in alcoholic drinks is ethanol, produced by. Why Is Ethanol In Alcohol.
From sciencenotes.org
Know the Difference Between Ethanol and Alcohol Why Is Ethanol In Alcohol alcohols, like water, are both weak bases and weak acids. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. Ethanol, or ethyl alcohol, is the only type of alcohol. In both pure water and pure ethanol the main intermolecular attractions are hydrogen. the distinction between alcohol and ethanol is. Why Is Ethanol In Alcohol.
From www.thoughtco.com
The Difference Between Alcohol and Ethanol Why Is Ethanol In Alcohol simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and are industrially synthesized in. consider ethanol as a typical small alcohol. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. There are two main processes for. In aqueous solutions, alcohols dissociate slightly by donating a. Why Is Ethanol In Alcohol.
From cartoondealer.com
Alcohol Ethanol, Ethyl Alcohol Molecule, Chemical Structure. Skeletal Why Is Ethanol In Alcohol consider ethanol as a typical small alcohol. the 'alcohol' in alcoholic drinks is ethanol, produced by the fermentation of sugars and found in wines, spirits and beers. There are two main processes for. the distinction between alcohol and ethanol is pretty simple: Ethanol, or ethyl alcohol, is the only type of alcohol. In both pure water and. Why Is Ethanol In Alcohol.
From zchemicals.com
Ethanol (Ethyl Alcohol), 200 proof, Denatured CDA19, 1 Gallon Z Why Is Ethanol In Alcohol ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. the distinction between alcohol and ethanol is pretty simple: There are two main processes for. alcohols, like water, are both weak bases and weak acids. simple alcohols like methanol, ethanol, and propanol occur in modest quantities in nature, and. Why Is Ethanol In Alcohol.
From thichuongtra.com
Is Ethanol A Natural Product? Exploring Its Origins And Properties Why Is Ethanol In Alcohol consider ethanol as a typical small alcohol. There are two main processes for. ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Ethanol, or ethyl alcohol, is the only type of alcohol. simple alcohols like methanol, ethanol,. Why Is Ethanol In Alcohol.