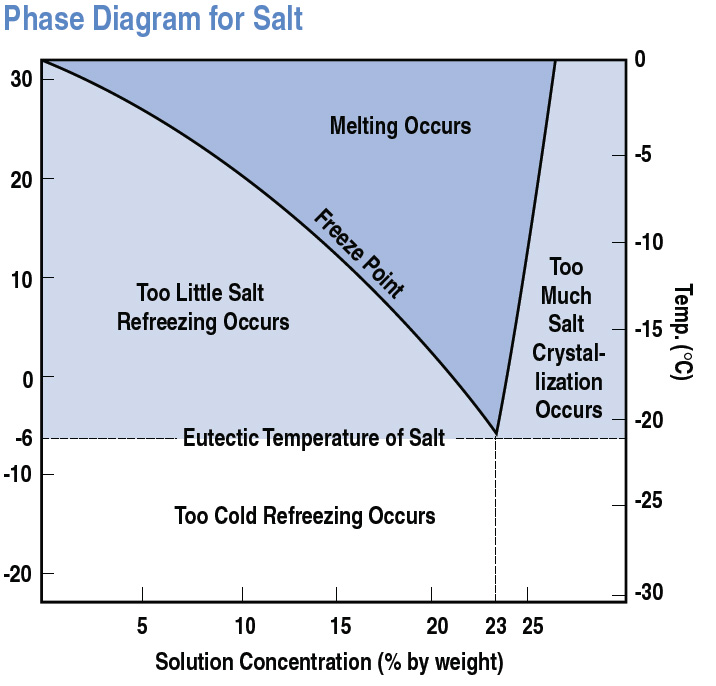
Rock Salt Activation Temperature . At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? An increase in confining pressure or grain size. An increase in either temperature or stress difference increases the creep rate considerably. It will melt ice, all the way down. Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm;
from meltsnow.com
An increase in confining pressure or grain size. Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm; Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). An increase in either temperature or stress difference increases the creep rate considerably. This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. It will melt ice, all the way down.
Salt vs. Temperature
Rock Salt Activation Temperature At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. An increase in either temperature or stress difference increases the creep rate considerably. It will melt ice, all the way down. An increase in confining pressure or grain size. Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm; Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the.
From slideplayer.com
Essential Question How does the temperature and salinity of water Rock Salt Activation Temperature It will melt ice, all the way down. Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm; At moderately high effective stress and temperatures in the range from 0. Rock Salt Activation Temperature.
From askfilo.com
If the density of a unit cell of a salt AB (rock salt type structure) is Rock Salt Activation Temperature It will melt ice, all the way down. At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? This paper investigates the influence of crystal orientation, temperature, deviatoric stress,. Rock Salt Activation Temperature.
From www.researchgate.net
Calculated DOS of rocksalt MgSe obtained from the optimal basis set IV Rock Salt Activation Temperature Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). At moderately high effective stress and temperatures in the range from 0 to 200. Rock Salt Activation Temperature.
From www.researchgate.net
FFT images of the (a) rocksalt and (b) layered areas. (c) HRTEM image Rock Salt Activation Temperature At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. An increase in either temperature or stress difference increases the creep rate considerably. Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? Besides evaporation, additional processes can lead. Rock Salt Activation Temperature.
From www.mdpi.com
Applied Sciences Free FullText Characteristics of Acoustic Rock Salt Activation Temperature An increase in either temperature or stress difference increases the creep rate considerably. This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? Measurements of steady state creep rates and activation. Rock Salt Activation Temperature.
From www.researchgate.net
Graphs σ 1 (ε 1 ) and V(ε 1 ) (a), (b) for rock salt and (c), (d) for Rock Salt Activation Temperature Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm; It will melt ice, all the way down. This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. An increase in confining pressure or grain size. Besides evaporation, additional. Rock Salt Activation Temperature.
From www.academia.edu
(PDF) Lowtemperature thermal expansion of rocksalt ZnO Tony Bell Rock Salt Activation Temperature This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. An increase in confining pressure or grain size. Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). Measurements of steady state creep. Rock Salt Activation Temperature.
From chlorideconscious.com
Best Application Rates For Rock Salt Rock Salt Activation Temperature It will melt ice, all the way down. Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? An increase in either temperature or. Rock Salt Activation Temperature.
From www.researchgate.net
Chemical analyses of rock salt, Baturn 1A. Download Scientific Diagram Rock Salt Activation Temperature An increase in either temperature or stress difference increases the creep rate considerably. This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984).. Rock Salt Activation Temperature.
From www.alamy.com
Chemical structure of Rock Salt Stock Vector Image & Art Alamy Rock Salt Activation Temperature An increase in either temperature or stress difference increases the creep rate considerably. This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. An increase in confining pressure or grain size. It will melt ice, all the way down. Besides evaporation, additional processes can lead to the formation of rock. Rock Salt Activation Temperature.
From www.researchgate.net
The absorption coefficient of AlN (rock salt, zinc blende, wurtzite Rock Salt Activation Temperature At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤. Rock Salt Activation Temperature.
From www.cargill.com
At What Temperature Does Rock Salt Stop Working? Cargill Rock Salt Activation Temperature Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. Measurements of steady state creep rates and activation parameters are described. Rock Salt Activation Temperature.
From www.researchgate.net
Freezing temperatures of salt brine mixtures Download Scientific Diagram Rock Salt Activation Temperature It will melt ice, all the way down. Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? An increase in either temperature or stress difference increases the creep rate considerably. Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines. Rock Salt Activation Temperature.
From www.alamy.com
Natural rock salt is the mineral form of sodium chloride called Halite Rock Salt Activation Temperature It will melt ice, all the way down. This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. An increase in either temperature or stress difference increases the creep rate considerably. Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective?. Rock Salt Activation Temperature.
From www.researchgate.net
Debye temperature and Sound velocities of AlN (rock salt, zinc blende Rock Salt Activation Temperature At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm; It will melt ice, all the way down. Besides evaporation, additional processes can lead to the formation. Rock Salt Activation Temperature.
From pubs.rsc.org
Azoniaspiro salts towards bridging the gap between roomtemperature Rock Salt Activation Temperature An increase in either temperature or stress difference increases the creep rate considerably. An increase in confining pressure or grain size. Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). It will melt ice, all the way down. Rock salt is a. Rock Salt Activation Temperature.
From www.slideserve.com
PPT Weathering PowerPoint Presentation, free download ID3755606 Rock Salt Activation Temperature At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. An increase in confining pressure or grain size. This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. Besides evaporation, additional processes can lead to the formation of. Rock Salt Activation Temperature.
From www.acs.org
Simulations & Videos for Lesson 5.6 Does Temperature Affect Dissolving Rock Salt Activation Temperature An increase in either temperature or stress difference increases the creep rate considerably. Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm; At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. Rock salt is a. Rock Salt Activation Temperature.
From www.mdpi.com
Materials Free FullText A Novel Interstitial Site in Binary Rock Rock Salt Activation Temperature Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). An increase in either temperature or stress difference increases the creep rate considerably. It will melt ice, all the way down. An increase in confining pressure or grain size. Measurements of steady state. Rock Salt Activation Temperature.
From sendhanamak.in
What is Rock Salt? its Benefits and Uses SendhaNamak Rock Salt Activation Temperature Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm; This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective?. Rock Salt Activation Temperature.
From nanohub.org
ECE 606 Lecture 2 Geometry of Periodic Crystals Rock Salt Activation Temperature Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm; An increase in either temperature or stress difference increases the creep rate considerably. This paper investigates the influence of crystal. Rock Salt Activation Temperature.
From www.degruyter.com
Characteristics and purification of Himalayan salt by high temperature Rock Salt Activation Temperature Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? An increase in either temperature or stress difference increases the creep rate considerably. At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. This paper investigates the influence of. Rock Salt Activation Temperature.
From www.researchgate.net
Value of the mass transfer coefficient in vertical surface of rock salt Rock Salt Activation Temperature Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of. Rock Salt Activation Temperature.
From en.climate-data.org
Salt Rock climate Average Temperature by month, Salt Rock water Rock Salt Activation Temperature Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. An increase in either temperature or stress difference increases the creep rate considerably. Measurements of steady state creep rates. Rock Salt Activation Temperature.
From www.slideserve.com
PPT Weathering PowerPoint Presentation, free download ID3755606 Rock Salt Activation Temperature At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). Measurements of steady state creep rates and activation parameters are described. Rock Salt Activation Temperature.
From www.researchgate.net
Effect of molten salt temperature on the conversion ratio Download Rock Salt Activation Temperature This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. An increase in confining pressure or grain size. At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. Measurements of steady state creep rates and activation parameters are. Rock Salt Activation Temperature.
From engineering.purdue.edu
JTRP Interaction of ChlorideBased Deicing Salts with Concrete Rock Salt Activation Temperature An increase in either temperature or stress difference increases the creep rate considerably. An increase in confining pressure or grain size. It will melt ice, all the way down. This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. At moderately high effective stress and temperatures in the range from. Rock Salt Activation Temperature.
From en.climate-data.org
Salt Rock climate Average Temperature by month, Salt Rock water Rock Salt Activation Temperature Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm; This paper investigates the influence of crystal orientation, temperature, deviatoric stress, and confining stress on the creep behavior of single. An increase in either temperature or stress difference increases the creep rate considerably. An increase in confining pressure or. Rock Salt Activation Temperature.
From boatcenter.netlify.app
Layered Rock Salt Structure Rock Salt Activation Temperature An increase in either temperature or stress difference increases the creep rate considerably. Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4. Rock Salt Activation Temperature.
From www.researchgate.net
Energyvolume curves of rock salt, hexagonal, wurtzite, and zinc blende Rock Salt Activation Temperature Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm; Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines. Rock Salt Activation Temperature.
From www.researchgate.net
(a) Cubic (room temperature) rock salt F m3m crystal structure of CoO Rock Salt Activation Temperature It will melt ice, all the way down. Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). An increase in confining pressure or. Rock Salt Activation Temperature.
From www.angi.com
Ice Melt vs. Rock Salt Pros, Cons, Safety Tips, and More Rock Salt Activation Temperature Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm; At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is. Rock Salt Activation Temperature.
From meltsnow.com
Salt vs. Temperature Rock Salt Activation Temperature Besides evaporation, additional processes can lead to the formation of rock salt, namely temperature changes (sloss, 1969), mixing of brines (raup, 1982), and brine freezing (sonnenfeld, 1984). An increase in confining pressure or grain size. At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. It will melt. Rock Salt Activation Temperature.
From www.researchgate.net
Ar irradiation of rocksalt GST films (a) Reflectance in the visible Rock Salt Activation Temperature An increase in confining pressure or grain size. Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? Measurements of steady state creep rates and activation parameters are described for rock salt at low temperature (t ≤ 0.4 tm; Besides evaporation, additional processes can lead to the formation of rock salt,. Rock Salt Activation Temperature.
From www.researchgate.net
The variation in ω(TA) with volume for rocksalt and zincblende phases Rock Salt Activation Temperature At moderately high effective stress and temperatures in the range from 0 to 200 \ (^\circ\) c, dislocation creep is generically the. Rock salt is a staple for most winter maintenance deicing programs, but at what temperature does it become ineffective? It will melt ice, all the way down. Measurements of steady state creep rates and activation parameters are described. Rock Salt Activation Temperature.