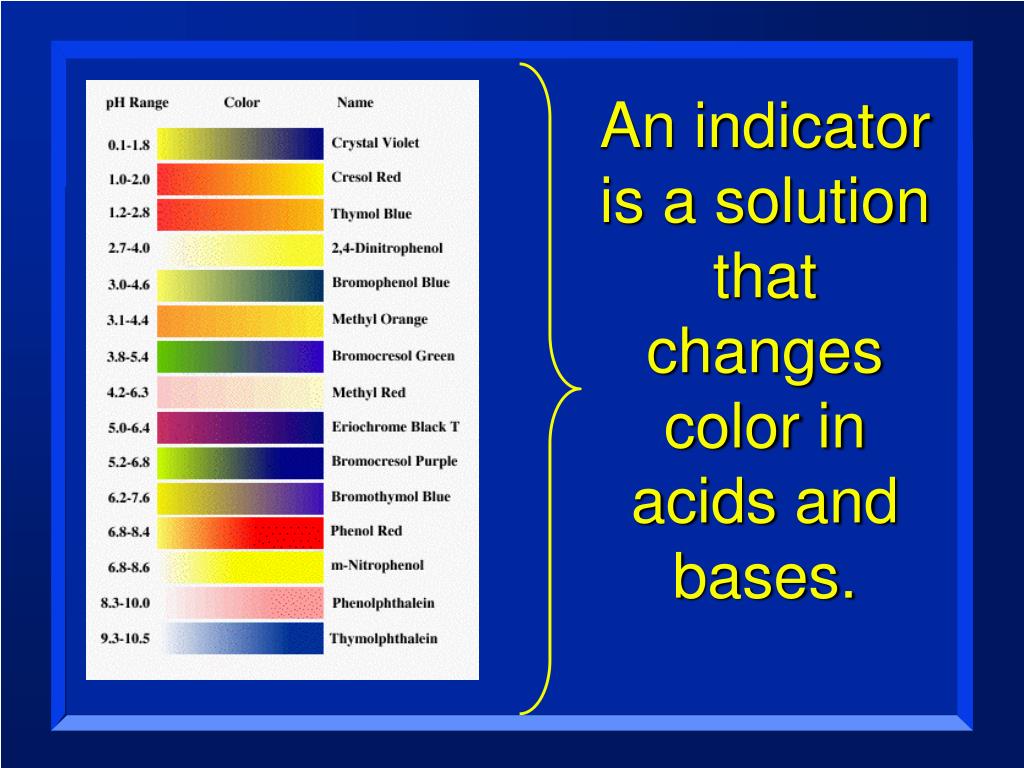
Indicator Colour Change In Acid And Base . Methyl orange or phenolphthalein would be less. Learn how to use indicators to classify acids, bases and salts based on their ph values. Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. Find out how to calculate the acid dissociation constant of indicators and the ph. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7.
from www.slideserve.com
Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. Methyl orange or phenolphthalein would be less. Find out how to calculate the acid dissociation constant of indicators and the ph. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. Learn how to use indicators to classify acids, bases and salts based on their ph values. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7.
PPT Properties of Acids PowerPoint Presentation, free download ID
Indicator Colour Change In Acid And Base The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. Methyl orange or phenolphthalein would be less. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. Learn how to use indicators to classify acids, bases and salts based on their ph values. Find out how to calculate the acid dissociation constant of indicators and the ph. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7.
From fphoto.photoshelter.com
science chemistry acid base indicator methyl orange Fundamental Indicator Colour Change In Acid And Base Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. Methyl orange or phenolphthalein would be less. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level.. Indicator Colour Change In Acid And Base.
From knowledge.carolina.com
AcidBase Indicators Carolina Knowledge Center Indicator Colour Change In Acid And Base Learn how to use indicators to classify acids, bases and salts based on their ph values. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Find out how to calculate the acid dissociation constant of indicators and the ph. Methyl. Indicator Colour Change In Acid And Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Colour Change In Acid And Base The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Learn how to use indicators to classify acids, bases and salts based. Indicator Colour Change In Acid And Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5741622 Indicator Colour Change In Acid And Base The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Methyl orange or phenolphthalein would be less. Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. Learn how to use indicators to. Indicator Colour Change In Acid And Base.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive Indicator Colour Change In Acid And Base Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. Methyl orange or phenolphthalein would be less. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis. Indicator Colour Change In Acid And Base.
From mavink.com
Ph Indicator Color Chart Indicator Colour Change In Acid And Base The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Find out how to calculate the acid dissociation constant of indicators and the ph. Methyl orange or phenolphthalein would be less. See examples of indicators such as litmus, phenolphthalein, and bromophenol. Indicator Colour Change In Acid And Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Colour Change In Acid And Base Find out how to calculate the acid dissociation constant of indicators and the ph. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. Learn how to use indicators to classify acids, bases and salts based on their ph values. Learn how indicators change color due to changes in ph, and how to determine. Indicator Colour Change In Acid And Base.
From www.thoughtco.com
Definition and Examples of AcidBase Indicator Indicator Colour Change In Acid And Base Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. Methyl orange or phenolphthalein would be less. Learn how to use indicators to classify acids, bases and salts based on their ph values. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level.. Indicator Colour Change In Acid And Base.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Indicator Colour Change In Acid And Base See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes. Indicator Colour Change In Acid And Base.
From www.vecteezy.com
Different indicators litmus, phenolpthalein, methyl orange in acid and Indicator Colour Change In Acid And Base Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how. Indicator Colour Change In Acid And Base.
From nigerianscholars.com
AcidBase Indicators AcidBase Equilibria Indicator Colour Change In Acid And Base Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. Methyl orange or phenolphthalein would be less. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. See examples of indicators such as. Indicator Colour Change In Acid And Base.
From learn.careers360.com
Make a list of various natural and synthetic indicators and write Indicator Colour Change In Acid And Base Methyl orange or phenolphthalein would be less. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Find out how to calculate the acid dissociation constant of indicators and the ph. Learn how indicators change color due to changes in ph,. Indicator Colour Change In Acid And Base.
From stock.adobe.com
Acidbase titration and phenolphthalein indicator Stock Vector Adobe Indicator Colour Change In Acid And Base Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Find out how to calculate the acid dissociation constant of indicators and the. Indicator Colour Change In Acid And Base.
From fphoto.photoshelter.com
science chemistry acid base indicator methyl red Fundamental Indicator Colour Change In Acid And Base Methyl orange or phenolphthalein would be less. Learn how to use indicators to classify acids, bases and salts based on their ph values. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Learn how. Indicator Colour Change In Acid And Base.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Indicator Colour Change In Acid And Base Find out how to calculate the acid dissociation constant of indicators and the ph. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Learn how to use indicators to classify acids, bases and salts. Indicator Colour Change In Acid And Base.
From www.slideserve.com
PPT AcidBase Equilibria PowerPoint Presentation, free download ID Indicator Colour Change In Acid And Base The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Methyl orange or phenolphthalein would be less. Learn how to use indicators to classify acids, bases and salts based on their ph values. Learn how indicators change color due to changes. Indicator Colour Change In Acid And Base.
From pediaa.com
Difference Between Acid Base Indicator and Universal Indicator Indicator Colour Change In Acid And Base Methyl orange or phenolphthalein would be less. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. Learn how to use indicators to. Indicator Colour Change In Acid And Base.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator Colour Change In Acid And Base The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Find out how to calculate the acid dissociation constant of indicators and the ph. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a. Indicator Colour Change In Acid And Base.
From fphoto.photoshelter.com
science chemistry acid base indicator methyl red Fundamental Indicator Colour Change In Acid And Base Find out how universal indicator changes colour in different solutions and how to measure ph accurately. Methyl orange or phenolphthalein would be less. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Find out how to calculate the acid dissociation. Indicator Colour Change In Acid And Base.
From mavink.com
Acid Base Indicator Colors Indicator Colour Change In Acid And Base See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Learn how indicators change color due to changes in ph, and how. Indicator Colour Change In Acid And Base.
From www.youtube.com
INDICATORS AND THEIR COLOUR CHANGES ACID BASE INDICATORS CHEMISTRY Indicator Colour Change In Acid And Base Methyl orange or phenolphthalein would be less. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Learn how indicators change color. Indicator Colour Change In Acid And Base.
From foundoutaboutchemistry.blogspot.com
Found Out About Chemistry Acidbase indicator charts Indicator Colour Change In Acid And Base Methyl orange or phenolphthalein would be less. Learn how to use indicators to classify acids, bases and salts based on their ph values. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Find out how universal indicator changes colour in. Indicator Colour Change In Acid And Base.
From www.slideserve.com
PPT PROPERTIES OF ACIDS & BASES PowerPoint Presentation, free Indicator Colour Change In Acid And Base Find out how universal indicator changes colour in different solutions and how to measure ph accurately. Find out how to calculate the acid dissociation constant of indicators and the ph. Learn how to use indicators to classify acids, bases and salts based on their ph values. Learn how indicators change color due to changes in ph, and how to determine. Indicator Colour Change In Acid And Base.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ Indicator Colour Change In Acid And Base Learn how to use indicators to classify acids, bases and salts based on their ph values. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Find out how universal indicator changes colour in different solutions and how to measure ph. Indicator Colour Change In Acid And Base.
From pt.slideshare.net
Acids & Bases Indicators Indicator Colour Change In Acid And Base Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. Find out how to calculate the acid dissociation constant of indicators and the ph. Methyl orange or phenolphthalein would be less. Learn how to use indicators. Indicator Colour Change In Acid And Base.
From davidswart.wordpress.com
Acids and Bases The Art of Science Indicator Colour Change In Acid And Base The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. Methyl orange or phenolphthalein would be less. Find out how to calculate the acid dissociation. Indicator Colour Change In Acid And Base.
From www.howtopronounce.com
Take the free online AcidBase Indicators Color Change! Chemistry Indicator Colour Change In Acid And Base Find out how to calculate the acid dissociation constant of indicators and the ph. Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Learn how to use indicators to classify acids, bases. Indicator Colour Change In Acid And Base.
From chimactiv.agroparistech.fr
Chimactiv Interactive numerical educational resources for the Indicator Colour Change In Acid And Base The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Methyl orange or phenolphthalein would be less. Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. Find out how universal indicator changes. Indicator Colour Change In Acid And Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Colour Change In Acid And Base Learn how to use indicators to classify acids, bases and salts based on their ph values. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Find out how universal indicator changes colour in different solutions and how to measure ph. Indicator Colour Change In Acid And Base.
From chem.libretexts.org
17.3 AcidBase Indicators Chemistry LibreTexts Indicator Colour Change In Acid And Base See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Learn how indicators change color due to changes in ph, and how. Indicator Colour Change In Acid And Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Colour Change In Acid And Base Methyl orange or phenolphthalein would be less. Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. See examples of indicators such as. Indicator Colour Change In Acid And Base.
From mungfali.com
Acid Base Titration Indicator Indicator Colour Change In Acid And Base Find out how universal indicator changes colour in different solutions and how to measure ph accurately. Learn how to use indicators to classify acids, bases and salts based on their ph values. Find out how to calculate the acid dissociation constant of indicators and the ph. Learn how indicators change color due to changes in ph, and how to determine. Indicator Colour Change In Acid And Base.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID Indicator Colour Change In Acid And Base Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation constants. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find out how to calculate the acid. Indicator Colour Change In Acid And Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Colour Change In Acid And Base The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Find out how to calculate the acid dissociation constant of indicators and the ph. Learn how indicators change color due to changes in ph, and how to determine their acidic dissociation. Indicator Colour Change In Acid And Base.
From sites.google.com
3 IB Chem 11 HL Whitehair Duarte Indicator Colour Change In Acid And Base Find out how to calculate the acid dissociation constant of indicators and the ph. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find out how universal indicator changes colour in different solutions and how to measure ph accurately. The litmus colour change happens over an unusually wide range, but. Indicator Colour Change In Acid And Base.