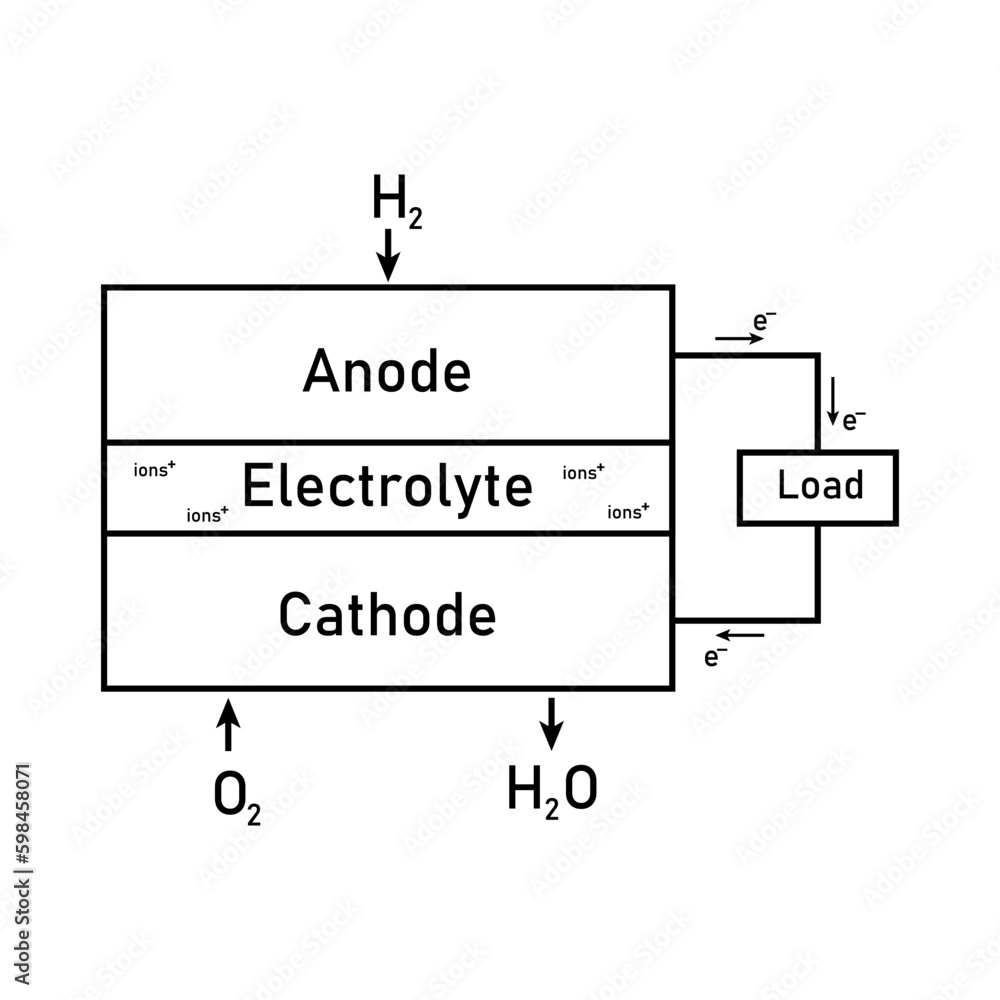
Hydrogen Fuel Cell Equation A Level . the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. fuel cells in a snap! Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode.
from stock.adobe.com
the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. fuel cells in a snap! revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen.
Block diagram of fuel cell. Schematic diagram of hydrogen fuel cell
Hydrogen Fuel Cell Equation A Level typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. fuel cells in a snap! the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum.
From www.youtube.com
AQA GCSE Chemistry P1 Hydrogen Fuel Cells YouTube Hydrogen Fuel Cell Equation A Level revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. the overall reaction in the fuel cell can be found by. Hydrogen Fuel Cell Equation A Level.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Hydrogen Fuel Cell Equation A Level revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. Hydrogen enters the cell through a porous carbon electrode which also contains. Hydrogen Fuel Cell Equation A Level.
From climatebiz.com
How To Build A DIY Hydrogen Fuel Cell Hydrogen Fuel Cell Equation A Level typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. revision notes on 5.4.6 fuel cells for the aqa a level chemistry. Hydrogen Fuel Cell Equation A Level.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Hydrogen Fuel Cell Equation A Level fuel cells in a snap! typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. Hydrogen enters the cell through a porous carbon electrode which also contains. Hydrogen Fuel Cell Equation A Level.
From www.researchgate.net
Schematic illustration of a hydrogen fuel cell. Download Scientific Hydrogen Fuel Cell Equation A Level the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. typically, in a hydrogen fuel cell, hydrogen gas (h2). Hydrogen Fuel Cell Equation A Level.
From climatebiz.com
How To Build A DIY Hydrogen Fuel Cell Hydrogen Fuel Cell Equation A Level the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. fuel cells in a snap! typically, in a. Hydrogen Fuel Cell Equation A Level.
From www.researchgate.net
Schematic diagram of hydrogen fuel cell Download Scientific Diagram Hydrogen Fuel Cell Equation A Level revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. . Hydrogen Fuel Cell Equation A Level.
From www.youtube.com
How does a hydrogen fuel cell work? (AKIO TV) YouTube Hydrogen Fuel Cell Equation A Level the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. fuel cells in a snap! revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. hydrogen—oxygen fuel cells are used. Hydrogen Fuel Cell Equation A Level.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Hydrogen Fuel Cell Equation A Level revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. the most. Hydrogen Fuel Cell Equation A Level.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Hydrogen Fuel Cell Equation A Level fuel cells in a snap! hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Hydrogen enters the cell through a porous carbon electrode which also. Hydrogen Fuel Cell Equation A Level.
From www.researchgate.net
Block diagram of a hydrogenbased fuel cell. Download Scientific Diagram Hydrogen Fuel Cell Equation A Level the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. fuel cells in a snap! Hydrogen enters the cell. Hydrogen Fuel Cell Equation A Level.
From www.numerade.com
SOLVED Describe a fuel cell. Include equations for the anode and Hydrogen Fuel Cell Equation A Level the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save. Hydrogen Fuel Cell Equation A Level.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记6.1.8 Fuel Cells翰林国际教育 Hydrogen Fuel Cell Equation A Level the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. . Hydrogen Fuel Cell Equation A Level.
From www.slideshare.net
Hydrogen fuel cells Hydrogen Fuel Cell Equation A Level the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written. Hydrogen Fuel Cell Equation A Level.
From dxoowccqh.blob.core.windows.net
How Do You Fill Hydrogen Fuel Cell at Gabriel Baer blog Hydrogen Fuel Cell Equation A Level revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. the overall. Hydrogen Fuel Cell Equation A Level.
From www.studypool.com
SOLUTION Hydrogen Fuel Cells IGCSE Chemistry Studypool Hydrogen Fuel Cell Equation A Level the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. fuel cells in a snap! Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while. Hydrogen Fuel Cell Equation A Level.
From www.researchgate.net
e Schematic view of a typical hydrogen PEM fuel cell and its components Hydrogen Fuel Cell Equation A Level the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors. Hydrogen Fuel Cell Equation A Level.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room Hydrogen Fuel Cell Equation A Level revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. the most common type of fuel cell is the hydrogen fuel. Hydrogen Fuel Cell Equation A Level.
From www.slideserve.com
PPT Fuel Cell Catalysts Based on Metal Nanoparticles PowerPoint Hydrogen Fuel Cell Equation A Level revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for. Hydrogen Fuel Cell Equation A Level.
From studylib.net
Module 6 Equation of State for Hydrogen Fuel Hydrogen Fuel Cell Equation A Level fuel cells in a snap! the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles. Hydrogen Fuel Cell Equation A Level.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Hydrogen Fuel Cell Equation A Level the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. the overall reaction in the fuel cell can be found by adding the two equations together, but because of the. Hydrogen Fuel Cell Equation A Level.
From large.stanford.edu
Hydrogen Fuel Cells Hydrogen Fuel Cell Equation A Level the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. fuel cells in a snap! hydrogen—oxygen fuel cells are used. Hydrogen Fuel Cell Equation A Level.
From www.sukorun.com
Hydrogen Fuel Cells Q&A Part 1 Sukorun Hydrogen Fuel Cell Equation A Level typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. fuel cells in a snap! hydrogen—oxygen fuel cells are used to. Hydrogen Fuel Cell Equation A Level.
From totalshield.com
Electrolyzer and Hydrogen Fuel Cell Safety TotalShield Hydrogen Fuel Cell Equation A Level Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the. Hydrogen Fuel Cell Equation A Level.
From www.nagwa.com
Question Video Combining Equations to Give the Overall Reaction for a Hydrogen Fuel Cell Equation A Level fuel cells in a snap! Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while. Hydrogen Fuel Cell Equation A Level.
From www.sundyne.com
Hydrogen Fuel Cells How Do They Work? Sundyne Hydrogen Fuel Cell Equation A Level revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. Hydrogen enters the cell through a porous carbon electrode which also contains. Hydrogen Fuel Cell Equation A Level.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Hydrogen Fuel Cell Equation A Level the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while. Hydrogen Fuel Cell Equation A Level.
From www.youtube.com
OCR C6 Fuel Cells (Higher) YouTube Hydrogen Fuel Cell Equation A Level fuel cells in a snap! hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. revision notes on 5.4.6 fuel cells for the. Hydrogen Fuel Cell Equation A Level.
From 640orfree.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip (2022) Hydrogen Fuel Cell Equation A Level fuel cells in a snap! hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at. Hydrogen Fuel Cell Equation A Level.
From mmerevise.co.uk
Commercial Applications of Electrochemical Cells MME Hydrogen Fuel Cell Equation A Level the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. the overall reaction in the fuel cell can be found by adding. Hydrogen Fuel Cell Equation A Level.
From www.nagwa.com
Question Video Identifying Which Equation Shows the Reaction at a Hydrogen Fuel Cell Equation A Level the overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons. fuel cells in a snap! hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. Hydrogen enters the cell through a porous carbon electrode. Hydrogen Fuel Cell Equation A Level.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube Hydrogen Fuel Cell Equation A Level hydrogen—oxygen fuel cells are used to provide electrical energy for electric motors in vehicles (a) (b) in a hydrogen—oxygen. the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written. Hydrogen Fuel Cell Equation A Level.
From www.youtube.com
Hydrogen & Fuel Cells Reactions Chemistry FuseSchool YouTube Hydrogen Fuel Cell Equation A Level fuel cells in a snap! typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Hydrogen enters the cell through a porous carbon electrode. Hydrogen Fuel Cell Equation A Level.
From chem.libretexts.org
2.2. Hydrogen, The Simplest Atom Chemistry LibreTexts Hydrogen Fuel Cell Equation A Level typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air. Hydrogen Fuel Cell Equation A Level.
From stock.adobe.com
Block diagram of fuel cell. Schematic diagram of hydrogen fuel cell Hydrogen Fuel Cell Equation A Level the most common type of fuel cell is the hydrogen fuel cell, which uses a continuous supply of hydrogen and oxygen from air to. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save. Hydrogen Fuel Cell Equation A Level.