Explain Calculation Of W Q Du . du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. Đq = 0 ideal gas ⇒. let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. U is a “state function” in. The first law of thermodynamics states that energy can be converted from one form to another with. Hence, the change in internal energy is. calculate q, w, du, and dh for a reversible adiabatic expansion in a closed system for one mole of an ideal monatomic gas. W is positive if work is done on the system. Reversible ⇒ du = cvdt. The heat q1 delivered to the gas makes it expand at constant temperature. q is positive as heat is added to the system.
from www.allaboutcircuits.com
q is positive as heat is added to the system. Reversible ⇒ du = cvdt. calculate q, w, du, and dh for a reversible adiabatic expansion in a closed system for one mole of an ideal monatomic gas. W is positive if work is done on the system. The first law of thermodynamics states that energy can be converted from one form to another with. Hence, the change in internal energy is. 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. Đq = 0 ideal gas ⇒. The heat q1 delivered to the gas makes it expand at constant temperature.
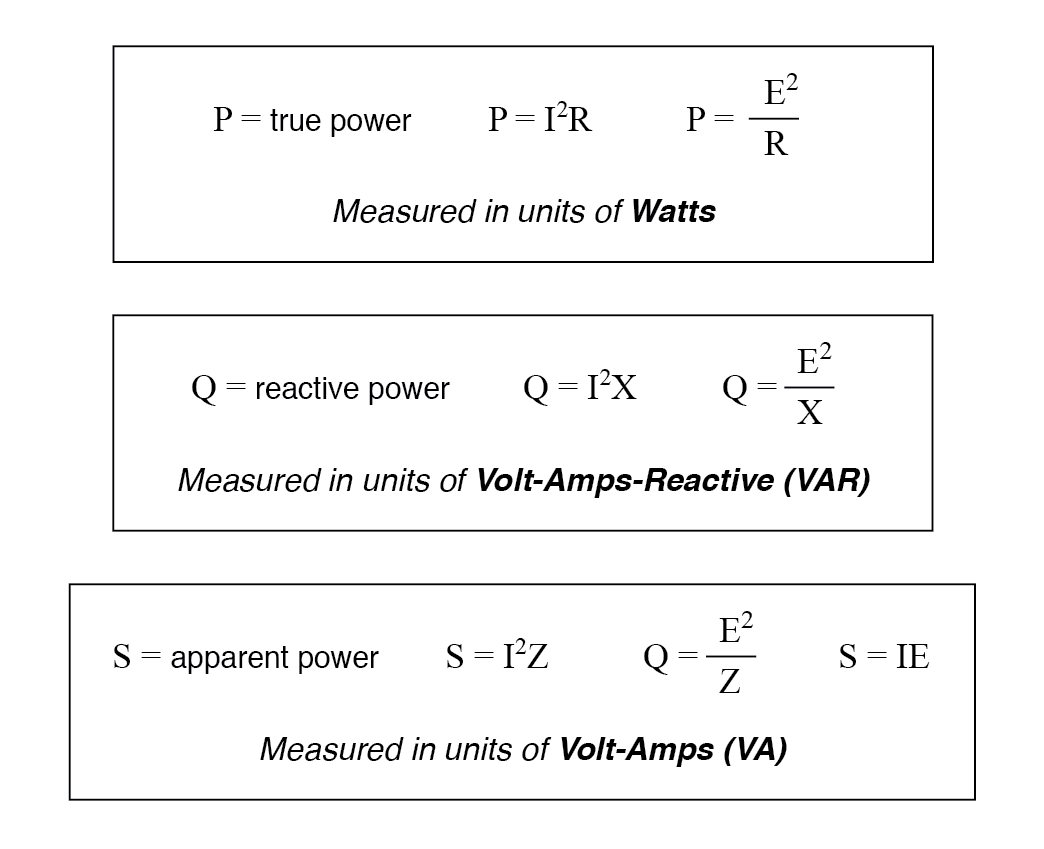
True, Reactive, and Apparent Power Power Factor Electronics Textbook
Explain Calculation Of W Q Du The first law of thermodynamics states that energy can be converted from one form to another with. calculate q, w, du, and dh for a reversible adiabatic expansion in a closed system for one mole of an ideal monatomic gas. U is a “state function” in. Đq = 0 ideal gas ⇒. Hence, the change in internal energy is. du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): Reversible ⇒ du = cvdt. q is positive as heat is added to the system. The heat q1 delivered to the gas makes it expand at constant temperature. W is positive if work is done on the system. 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. The first law of thermodynamics states that energy can be converted from one form to another with.
From dokumen.tips
(PDF) LIFECON DELIVERABLE D 5.3 Methodology and …lifecon.vtt.fi/d53.pdf Explain Calculation Of W Q Du Hence, the change in internal energy is. q is positive as heat is added to the system. let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): du = δq + δw δu = q + w (1.71) these equations are. Explain Calculation Of W Q Du.
From www.allaboutcircuits.com
True, Reactive, and Apparent Power Power Factor Electronics Textbook Explain Calculation Of W Q Du W is positive if work is done on the system. U is a “state function” in. The heat q1 delivered to the gas makes it expand at constant temperature. 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. q is positive as heat is added to the system. Đq = 0 ideal gas ⇒. Reversible ⇒. Explain Calculation Of W Q Du.
From exojrzcfd.blob.core.windows.net
Price Elasticity Of Supply And Its Types at William Wheeler blog Explain Calculation Of W Q Du The heat q1 delivered to the gas makes it expand at constant temperature. q is positive as heat is added to the system. Reversible ⇒ du = cvdt. Đq = 0 ideal gas ⇒. The first law of thermodynamics states that energy can be converted from one form to another with. Hence, the change in internal energy is. . Explain Calculation Of W Q Du.
From www.toppr.com
Calculate Q,w,Δ U,Δ H for the isothermal reversible expansion of 1 mole Explain Calculation Of W Q Du Đq = 0 ideal gas ⇒. 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. Reversible ⇒ du = cvdt. calculate q, w, du, and dh for a reversible adiabatic expansion in a closed system for one mole of an ideal monatomic gas. U is a “state function” in. The heat q1 delivered to the gas. Explain Calculation Of W Q Du.
From www.chegg.com
Solved Calculation of the magnitudes (q, w, DU and DH) and Explain Calculation Of W Q Du 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): du = δq + δw δu = q + w (1.71) these equations are also known as the first law. Explain Calculation Of W Q Du.
From www.researchgate.net
Entries of scale matrix function W (q) Download Scientific Diagram Explain Calculation Of W Q Du du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. q is positive as heat is added to the system. The heat q1 delivered to the gas makes it expand at constant temperature. U is a “state function” in. Đq = 0 ideal gas ⇒. . Explain Calculation Of W Q Du.
From www.chegg.com
Solved Let W and Q be two bivariate normal random variables Explain Calculation Of W Q Du 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. Reversible ⇒ du = cvdt. The heat q1 delivered to the gas makes it expand at constant temperature. du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. q is positive as heat is. Explain Calculation Of W Q Du.
From www.researchgate.net
The potential W (q) for k \ 1 and for c \ 1, 2, 4.8, 6. Download Explain Calculation Of W Q Du q is positive as heat is added to the system. The heat q1 delivered to the gas makes it expand at constant temperature. calculate q, w, du, and dh for a reversible adiabatic expansion in a closed system for one mole of an ideal monatomic gas. The first law of thermodynamics states that energy can be converted from. Explain Calculation Of W Q Du.
From www.chegg.com
Solved Suppose a firm has the following production function Explain Calculation Of W Q Du Reversible ⇒ du = cvdt. du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. The first law of thermodynamics states that energy can be converted from one form to another with. U is a “state function” in. Đq = 0 ideal gas ⇒. 1 mole. Explain Calculation Of W Q Du.
From twitter.com
Chelsea ParlettPelleriti on Twitter "In transformers, for a single Explain Calculation Of W Q Du 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. Reversible ⇒ du = cvdt. The first law of thermodynamics states that energy can be converted from one form to another with. q is. Explain Calculation Of W Q Du.
From www.trendradars.com
Wheel Of Time's Natasha O'Keeffe Teases The Evolution Of Lanfear In Explain Calculation Of W Q Du Reversible ⇒ du = cvdt. U is a “state function” in. let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): W is positive if work is done on the system. Hence, the change in internal energy is. du = δq +. Explain Calculation Of W Q Du.
From www.slideserve.com
PPT Fig. 1711 The net work done by the system in the process aba is Explain Calculation Of W Q Du q is positive as heat is added to the system. The first law of thermodynamics states that energy can be converted from one form to another with. du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. Hence, the change in internal energy is. W is. Explain Calculation Of W Q Du.
From ppt-online.org
Термодинамика диэлектриков. Типы диэлектриков, свойства и применение Explain Calculation Of W Q Du du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. q is positive as heat is added to the system. The first law of thermodynamics states that energy can be converted from one form to another with. let us calculate the work done by a. Explain Calculation Of W Q Du.
From www.researchgate.net
Loglog plot of dU (q)/dq at q = qc versus L. The solid line is the Explain Calculation Of W Q Du U is a “state function” in. W is positive if work is done on the system. Reversible ⇒ du = cvdt. du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. The heat q1 delivered to the gas makes it expand at constant temperature. q is. Explain Calculation Of W Q Du.
From www.youtube.com
How to calculate Qp/Qs? Hatem Hosny YouTube Explain Calculation Of W Q Du Hence, the change in internal energy is. 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. The first law of thermodynamics states that energy can be converted from one form to another with. The heat q1 delivered to the gas makes it expand at constant temperature. q is positive as heat is added to the system.. Explain Calculation Of W Q Du.
From www.researchgate.net
Compatibility of Q w /Q 1 for Schmidt Approach (present study), Eq. 18 Explain Calculation Of W Q Du q is positive as heat is added to the system. let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): The heat q1 delivered to the gas makes it expand at constant temperature. 1 mole gas (v1,t1) = 1 mole gas. Explain Calculation Of W Q Du.
From theinstrumentguru.com
Control valve sizing calculator Cv Calculator Explain Calculation Of W Q Du 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. q is positive as heat is added to the system. W is positive if work is done on the system. The heat q1 delivered to the gas makes it expand at constant temperature. U is a “state function” in. Đq = 0 ideal gas ⇒. du. Explain Calculation Of W Q Du.
From www.researchgate.net
Convective flux w q (kW/m 2 ) distribution for X33 upper part, Mach Explain Calculation Of W Q Du W is positive if work is done on the system. Đq = 0 ideal gas ⇒. U is a “state function” in. du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. let us calculate the work done by a mole of an ideal gas in. Explain Calculation Of W Q Du.
From www.toppr.com
Fro each of the following system and time intervals write the Explain Calculation Of W Q Du q is positive as heat is added to the system. Reversible ⇒ du = cvdt. Hence, the change in internal energy is. The heat q1 delivered to the gas makes it expand at constant temperature. let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to. Explain Calculation Of W Q Du.
From www.slideserve.com
PPT Thermodynamics Temperature, Heat Transfer, and First Law of Explain Calculation Of W Q Du The first law of thermodynamics states that energy can be converted from one form to another with. let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): Reversible ⇒ du = cvdt. The heat q1 delivered to the gas makes it expand at. Explain Calculation Of W Q Du.
From www.bestbuy.com
Samsung Q series 5.1.2ch Wireless Dolby Atmos Soundbar w/ Q Symphony Explain Calculation Of W Q Du U is a “state function” in. W is positive if work is done on the system. let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): Hence, the change in internal energy is. Đq = 0 ideal gas ⇒. q is positive. Explain Calculation Of W Q Du.
From physics.stackexchange.com
thermodynamics \delta Q = dU + \delta W. Why is it dU while Explain Calculation Of W Q Du W is positive if work is done on the system. Reversible ⇒ du = cvdt. Đq = 0 ideal gas ⇒. let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): du = δq + δw δu = q + w (1.71). Explain Calculation Of W Q Du.
From www.researchgate.net
This calculation is faulty although the result is perfect. It should be Explain Calculation Of W Q Du U is a “state function” in. The first law of thermodynamics states that energy can be converted from one form to another with. 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. q is positive as heat is added to the system. Hence, the change in internal energy is. The heat q1 delivered to the gas. Explain Calculation Of W Q Du.
From www.studocu.com
QRM 21 and 18 merged 1 / 2 1 / F (x) = c(p, q) Z x 0 u p (1 u) q du Explain Calculation Of W Q Du Hence, the change in internal energy is. Đq = 0 ideal gas ⇒. 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. Reversible ⇒ du = cvdt. q is positive as heat is added to the system. let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion. Explain Calculation Of W Q Du.
From www.slideserve.com
PPT แบบฝึกหัด หนังสือทบวง PowerPoint Presentation ID4409295 Explain Calculation Of W Q Du du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. Reversible ⇒ du = cvdt. U is a “state function” in. calculate q, w, du, and dh for a reversible adiabatic expansion in a closed system for one mole of an ideal monatomic gas. The heat. Explain Calculation Of W Q Du.
From www.youtube.com
Calculate Q,W,ΔU,ΔH for a Double Process YouTube Explain Calculation Of W Q Du calculate q, w, du, and dh for a reversible adiabatic expansion in a closed system for one mole of an ideal monatomic gas. Reversible ⇒ du = cvdt. Đq = 0 ideal gas ⇒. Hence, the change in internal energy is. The heat q1 delivered to the gas makes it expand at constant temperature. q is positive as. Explain Calculation Of W Q Du.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID4494868 Explain Calculation Of W Q Du let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): The heat q1 delivered to the gas makes it expand at constant temperature. Đq = 0 ideal gas ⇒. U is a “state function” in. The first law of thermodynamics states that energy. Explain Calculation Of W Q Du.
From math.stackexchange.com
orthogonality Proving (Qv) \cdot (Qw) = v \cdot w given Q is Explain Calculation Of W Q Du du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. Hence, the change in internal energy is. The first law of thermodynamics states that energy can be converted from one form to another with. Reversible ⇒ du = cvdt. Đq = 0 ideal gas ⇒. let. Explain Calculation Of W Q Du.
From askfilo.com
The expressions that relate (i) Q,I and t and (ii) Q,V and W respectively.. Explain Calculation Of W Q Du Reversible ⇒ du = cvdt. calculate q, w, du, and dh for a reversible adiabatic expansion in a closed system for one mole of an ideal monatomic gas. 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. Hence, the change in internal energy is. Đq = 0 ideal gas ⇒. The first law of thermodynamics states. Explain Calculation Of W Q Du.
From www.researchgate.net
Distributions of the reconstructed W q W q and Z q Z q events at the m Explain Calculation Of W Q Du The heat q1 delivered to the gas makes it expand at constant temperature. Hence, the change in internal energy is. Reversible ⇒ du = cvdt. W is positive if work is done on the system. q is positive as heat is added to the system. 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. du. Explain Calculation Of W Q Du.
From www.quora.com
What are the formulas and units of Q , Q ̇ and q ̇ in heat transfer Explain Calculation Of W Q Du du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. The heat q1 delivered to the gas makes it expand at constant temperature. U is a “state function” in. 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. Reversible ⇒ du = cvdt. . Explain Calculation Of W Q Du.
From scoop.eduncle.com
The degree of unsaturation (double bond equivalent) for a compound with Explain Calculation Of W Q Du The first law of thermodynamics states that energy can be converted from one form to another with. du = δq + δw δu = q + w (1.71) these equations are also known as the first law of thermodynamics. q is positive as heat is added to the system. calculate q, w, du, and dh for a. Explain Calculation Of W Q Du.
From www.scribd.com
Chapter 4 Creating Customer Value Satisfaction and Loyalty W Q Explain Calculation Of W Q Du U is a “state function” in. let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): calculate q, w, du, and dh for a reversible adiabatic expansion in a closed system for one mole of an ideal monatomic gas. W is positive. Explain Calculation Of W Q Du.
From slideplayer.com
First Law of Thermodynamics ppt download Explain Calculation Of W Q Du 1 mole gas (v1,t1) = 1 mole gas (v2,t2) adiabatic ⇒. Reversible ⇒ du = cvdt. calculate q, w, du, and dh for a reversible adiabatic expansion in a closed system for one mole of an ideal monatomic gas. Đq = 0 ideal gas ⇒. U is a “state function” in. W is positive if work is done. Explain Calculation Of W Q Du.
From www.researchgate.net
The detailed circuits for W, Q, and V cells. Download Scientific Diagram Explain Calculation Of W Q Du let us calculate the work done by a mole of an ideal gas in a reversible adiabatic expansion from (p1 , v1) to (p2 , v2): calculate q, w, du, and dh for a reversible adiabatic expansion in a closed system for one mole of an ideal monatomic gas. The heat q1 delivered to the gas makes it. Explain Calculation Of W Q Du.