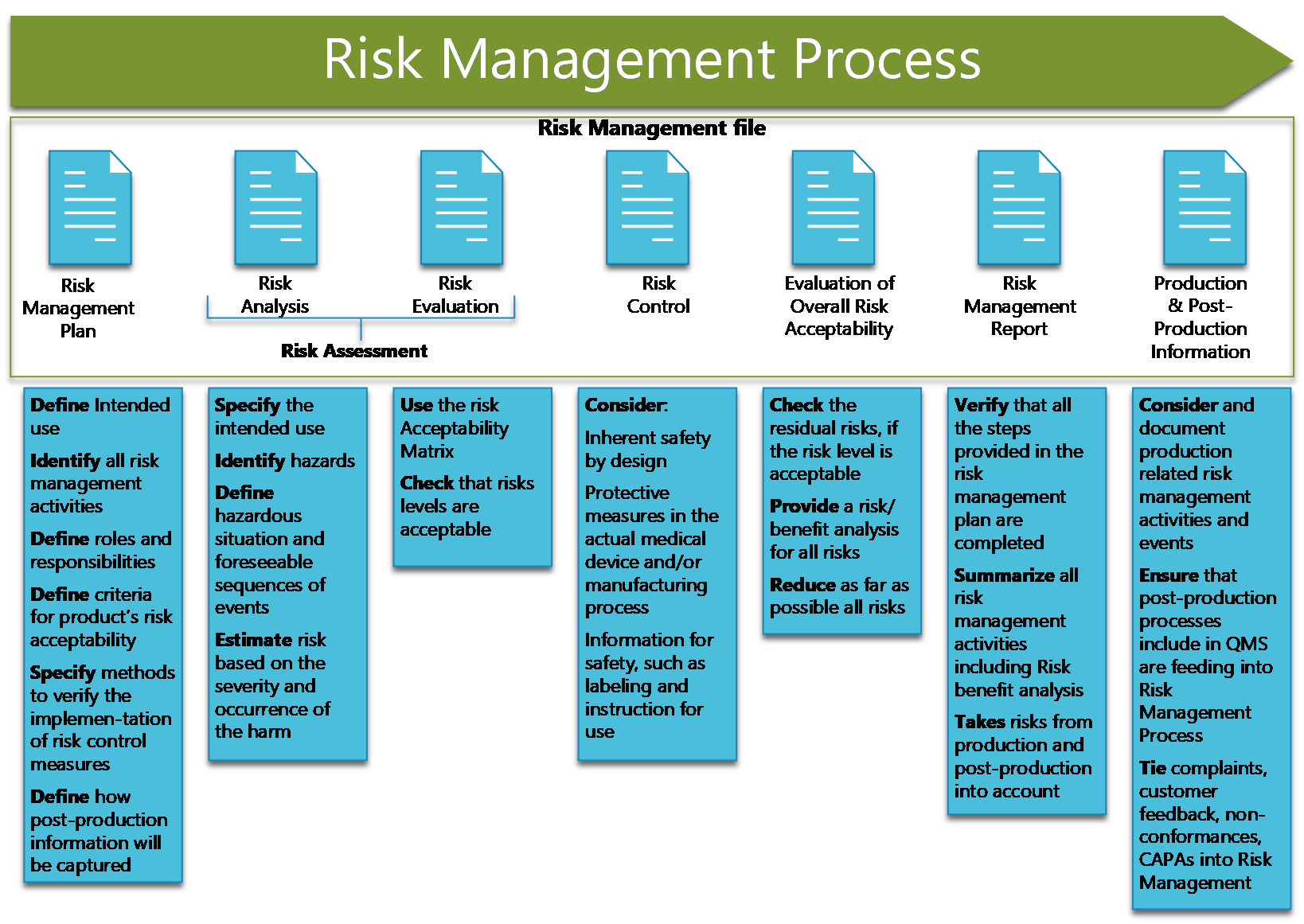
Medical Devices Management . A free brochure with tips for getting started with iso 13485, requirements for quality. medical devices are products or equipment intended for a medical purpose. Medical devices in particular are crucial for. In the european union (eu) they must undergo a. Health technologies are essential for a functioning health system. • management of medical devices •. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. management and safe use of medical devices.
from exoaernau.blob.core.windows.net
quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. • management of medical devices •. medical devices are products or equipment intended for a medical purpose. Health technologies are essential for a functioning health system. A free brochure with tips for getting started with iso 13485, requirements for quality. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. In the european union (eu) they must undergo a. Medical devices in particular are crucial for. management and safe use of medical devices.
Medical Device Risk Management Documents at Terrence Knapp blog
Medical Devices Management quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. medical devices are products or equipment intended for a medical purpose. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. In the european union (eu) they must undergo a. Medical devices in particular are crucial for. Health technologies are essential for a functioning health system. A free brochure with tips for getting started with iso 13485, requirements for quality. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. management and safe use of medical devices. • management of medical devices •.
From www.orielstat.com
Medical Device QMS 101 What It Is, Where It’s Required, and Key Medical Devices Management iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. • management of medical devices •. medical devices are products or equipment intended for a medical purpose. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet. Medical Devices Management.
From simbex.com
ISO 14971 Managing Risk in Medical Device Development Medical Devices Management management and safe use of medical devices. A free brochure with tips for getting started with iso 13485, requirements for quality. medical devices are products or equipment intended for a medical purpose. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. Medical devices in particular are crucial for. • management. Medical Devices Management.
From www.scribd.com
Clcgp004 Medical Devices Governance Management Policy v6 Medical Medical Devices Management management and safe use of medical devices. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. Medical devices in particular are crucial for. medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a. Health technologies are essential for a functioning. Medical Devices Management.
From timly.com
Medical Device Management in Healthcare Sector Made Easy Medical Devices Management In the european union (eu) they must undergo a. • management of medical devices •. A free brochure with tips for getting started with iso 13485, requirements for quality. medical devices are products or equipment intended for a medical purpose. Medical devices in particular are crucial for. management and safe use of medical devices. iso 13485:2016 specifies. Medical Devices Management.
From www.nbautomation.com
Medical devices' management and traceability NB Automation Medical Devices Management A free brochure with tips for getting started with iso 13485, requirements for quality. medical devices are products or equipment intended for a medical purpose. • management of medical devices •. Health technologies are essential for a functioning health system. management and safe use of medical devices. iso 13485:2016 specifies requirements for a quality management system where. Medical Devices Management.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Medical Devices Management management and safe use of medical devices. Health technologies are essential for a functioning health system. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. medical devices are products or equipment intended for a medical purpose. medical devices are. Medical Devices Management.
From www.ebme.co.uk
Medical devices getting management policies right Medical Devices Management iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. management and safe use of medical devices. Medical devices in particular are crucial for. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. A. Medical Devices Management.
From blogs.dctc.edu
Program Spotlight Biomedical Equipment Technology » DCTC News Medical Devices Management A free brochure with tips for getting started with iso 13485, requirements for quality. medical devices are products or equipment intended for a medical purpose. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. Medical devices in particular are crucial for. management and safe use of medical devices. In the. Medical Devices Management.
From www.syscreations.com
Medical Device Integration Solutions With EMR, EHR, LIS, HIS Medical Devices Management Medical devices in particular are crucial for. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. medical devices are products or equipment intended for a medical purpose. medical devices are used in many diverse settings, for example, by laypersons at. Medical Devices Management.
From www.elinext.com
Medical Practice Management Software in a Nutshell Elinext Medical Devices Management quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. In the european union (eu) they must undergo a. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. medical devices are used in many. Medical Devices Management.
From www.iso-certs.co.uk
MEDICAL DEVICES MANAGEMENT SYSTEM ISO CERT INTERNATIONAL LTD Medical Devices Management medical devices are products or equipment intended for a medical purpose. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. In the european union (eu) they must undergo a. Medical devices in particular. Medical Devices Management.
From www.greenlight.guru
Ultimate Guide to ISO 13485 Quality Management System (QMS) for Medical Medical Devices Management • management of medical devices •. In the european union (eu) they must undergo a. medical devices are products or equipment intended for a medical purpose. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. Medical devices in particular are crucial for. Health technologies are essential for a functioning health system.. Medical Devices Management.
From operonstrategist.com
Software as a Medical Device (SaMD) IEC 62304 Certification Medical Devices Management • management of medical devices •. medical devices are products or equipment intended for a medical purpose. management and safe use of medical devices. Health technologies are essential for a functioning health system. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. iso 13485:2016 specifies requirements for a. Medical Devices Management.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Devices Management Health technologies are essential for a functioning health system. A free brochure with tips for getting started with iso 13485, requirements for quality. • management of medical devices •. management and safe use of medical devices. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective,. Medical Devices Management.
From sunstonepilot.com
Introduction to Medical Device Development Sunstone Pilot, Inc. Medical Devices Management management and safe use of medical devices. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. Medical devices in particular are crucial for. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements.. Medical Devices Management.
From www.iso-certs.co.uk
ISO 13485 MEDICAL DEVICES MANAGEMENT SYSTEM ISO CERT INTERNATIONAL LTD Medical Devices Management quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. • management of medical devices •. medical devices are products or equipment intended for a medical purpose. A free brochure with tips for getting started with iso 13485, requirements for quality. . Medical Devices Management.
From www.exsolutiongroup.com
ISO 134852003 Quality Management System for Medical Devices QMS for Medical Devices Management medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. In the european union (eu) they must undergo a. Health technologies are essential for a functioning health system. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and. Medical Devices Management.
From www.universityofgalway.ie
Medical Device Management University of Galway Medical Devices Management medical devices are products or equipment intended for a medical purpose. Medical devices in particular are crucial for. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective,. Medical Devices Management.
From www.birlasoft.com
Acing Master Data Management in Medical Device Industry Medical Devices Management • management of medical devices •. A free brochure with tips for getting started with iso 13485, requirements for quality. Health technologies are essential for a functioning health system. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. medical devices are products or equipment intended for a medical purpose. . Medical Devices Management.
From pharmait.dk
Medical Device Quality Management System processes Pharma IT Medical Devices Management Health technologies are essential for a functioning health system. A free brochure with tips for getting started with iso 13485, requirements for quality. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. management and safe use of medical devices. medical devices are products or equipment intended for a medical. Medical Devices Management.
From www.slideshare.net
6 Ways to Improve Your Medical Device Management Medical Devices Management medical devices are products or equipment intended for a medical purpose. A free brochure with tips for getting started with iso 13485, requirements for quality. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate. Medical Devices Management.
From medicaldevicehq.com
The Perfect Project Process Medical Device Product Development Medical Devices Management A free brochure with tips for getting started with iso 13485, requirements for quality. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. Medical devices in particular are crucial for. Health technologies are essential for a functioning health system. iso 13485:2016. Medical Devices Management.
From www.scribd.com
Lecture2 Introduction To Medical Devices Management PDF Medical Devices Management • management of medical devices •. Health technologies are essential for a functioning health system. management and safe use of medical devices. medical devices are products or equipment intended for a medical purpose. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. Medical devices in particular are crucial for.. Medical Devices Management.
From exoaernau.blob.core.windows.net
Medical Device Risk Management Documents at Terrence Knapp blog Medical Devices Management medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. A free brochure with tips for getting started with iso 13485, requirements for quality. management and safe use of medical devices. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices. Medical Devices Management.
From edgeiq.blogspot.com
EdgeIQ Complete Medical Device Management Solution with EdgeIQ Medical Devices Management Medical devices in particular are crucial for. Health technologies are essential for a functioning health system. In the european union (eu) they must undergo a. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff.. Medical Devices Management.
From www.youtube.com
Automating Medical Device Management YouTube Medical Devices Management quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. A free brochure with tips for getting started with iso 13485, requirements for quality. Medical devices in particular are crucial for. medical devices are used in many diverse settings, for example, by. Medical Devices Management.
From www.researchgate.net
The LifeCycle of a Medical Device. Download Scientific Diagram Medical Devices Management management and safe use of medical devices. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. In the european union (eu) they must undergo a. Medical devices in particular are crucial for. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. Health. Medical Devices Management.
From enterprisepeak.com
Medical Devices from Concept to Commercialization Enterprise Peak Medical Devices Management In the european union (eu) they must undergo a. medical devices are products or equipment intended for a medical purpose. Health technologies are essential for a functioning health system. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. A free brochure. Medical Devices Management.
From www.jli.edu.in
5 Phases of Medical Device Development Process JLI Blog Medical Devices Management A free brochure with tips for getting started with iso 13485, requirements for quality. management and safe use of medical devices. Health technologies are essential for a functioning health system. • management of medical devices •. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective,. Medical Devices Management.
From www.slideteam.net
Medical Device Development And Commercialization Process Medical Devices Management Health technologies are essential for a functioning health system. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. A free brochure with tips for. Medical Devices Management.
From www.researchgate.net
Medical device lifecycle management (see online version for colours Medical Devices Management Health technologies are essential for a functioning health system. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. In the european union (eu) they must undergo a. Medical devices in particular are crucial for.. Medical Devices Management.
From www.cognidox.com
4 ways to build a medical device quality management system Medical Devices Management • management of medical devices •. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. management and safe use of medical devices. Medical devices in particular are crucial for. A free brochure with tips for getting started with iso 13485, requirements for quality. medical devices are products or equipment. Medical Devices Management.
From sunstonepilot.com
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc. Medical Devices Management • management of medical devices •. Health technologies are essential for a functioning health system. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. management and safe use of medical devices. iso 13485:2016 specifies requirements for a quality management system. Medical Devices Management.
From www.joharidigital.com
Understanding The 7 Phases of Medical Device Development & Manufacturing Medical Devices Management quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. medical devices are products or equipment intended for a medical purpose. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its. • management of medical. Medical Devices Management.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Medical Devices Management quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory requirements. • management of medical devices •. medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff. iso 13485:2016 specifies requirements for a quality management. Medical Devices Management.