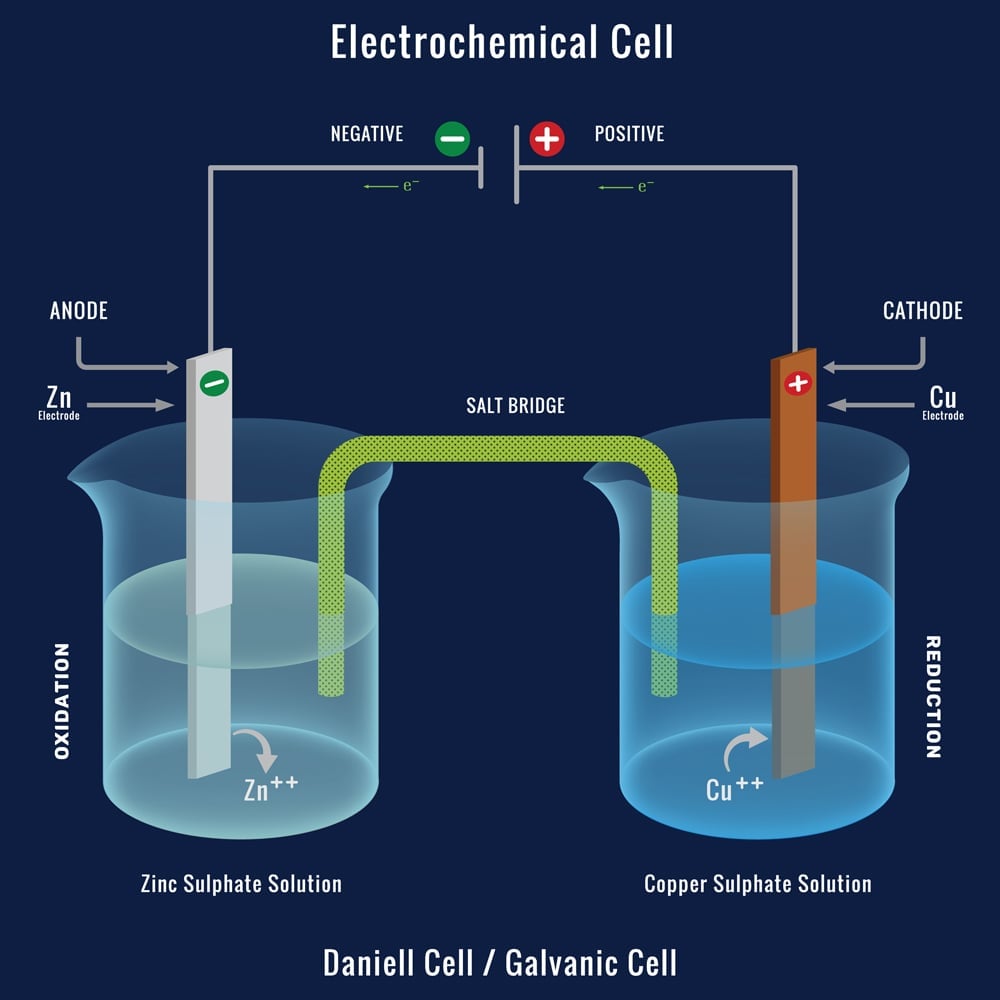
Zinc And Copper Galvanic Cell . To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. From the information given in. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. In the process of the reaction,. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current.
from www.scienceabc.com
From the information given in. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. In the process of the reaction,. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates.
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC
Zinc And Copper Galvanic Cell From the information given in. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. In the process of the reaction,. From the information given in.
From www.youtube.com
Electrochemical cell Zn and Cu YouTube Zinc And Copper Galvanic Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. In the process of the reaction,. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. From. Zinc And Copper Galvanic Cell.
From byjus.com
Daniell Cell Definition, Construction & Working with Cell Reactions Zinc And Copper Galvanic Cell One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. In the process of the reaction,. Galvanic cell with zinc and copper depicts a voltaic cell and. Zinc And Copper Galvanic Cell.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure including zinc anode copper Zinc And Copper Galvanic Cell From the information given in. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the. Zinc And Copper Galvanic Cell.
From www.alamy.com
Daniell element galvanic cell with zinc and copper Stock Photo, Royalty Free Image 116479798 Zinc And Copper Galvanic Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of. Zinc And Copper Galvanic Cell.
From thegoodchemistguide.blogspot.com
The Good Chemist's Guide Copper Zinc Galvanic Cell Zinc And Copper Galvanic Cell To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. In the process of the reaction,. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. Use cell notation to describe the galvanic cell where copper(ii) ions. Zinc And Copper Galvanic Cell.
From cider.uoregon.edu
Electrochemcial Cell Demonstration Voltaic Cell Zinc/Copper CIDER Zinc And Copper Galvanic Cell Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. From the information given in. One reactant gives up electrons (undergoes oxidation) and another. Zinc And Copper Galvanic Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2810901 Zinc And Copper Galvanic Cell From the information given in. In the process of the reaction,. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A simple electrochemical cell can be made from copper and zinc metals with. Zinc And Copper Galvanic Cell.
From chem.libretexts.org
14.1 Cell Diagrams and Cell Reactions Chemistry LibreTexts Zinc And Copper Galvanic Cell In the process of the reaction,. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic. Zinc And Copper Galvanic Cell.
From www.youtube.com
COMPONENTS OF A ZINC COPPER VOLTAIC CELL YouTube Zinc And Copper Galvanic Cell In the process of the reaction,. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. Galvanic cell with zinc and copper. Zinc And Copper Galvanic Cell.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and copper is the cathode Zinc And Copper Galvanic Cell One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. In the process of the reaction,. To illustrate the. Zinc And Copper Galvanic Cell.
From www1.chem.umn.edu
CopperZinc Galvanic Cell Zinc And Copper Galvanic Cell A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. From the information given in. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. Use cell notation to describe the galvanic. Zinc And Copper Galvanic Cell.
From www.freepik.com
Premium Photo Electrochemical cell or Galvanic cell. The Daniell cell is a primary voltaic Zinc And Copper Galvanic Cell In the process of the reaction,. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. To illustrate the basic principles of a galvanic. Zinc And Copper Galvanic Cell.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube Zinc And Copper Galvanic Cell One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. In the process of the reaction,. From the information. Zinc And Copper Galvanic Cell.
From mavink.com
Zinc Copper Galvanic Cell Zinc And Copper Galvanic Cell From the information given in. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal. Zinc And Copper Galvanic Cell.
From www.slideserve.com
PPT Chapter 22 Electrochemistry PowerPoint Presentation, free download ID795164 Zinc And Copper Galvanic Cell A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. Use cell notation to describe the galvanic cell where copper(ii) ions are. Zinc And Copper Galvanic Cell.
From chempedia.info
Zinccopper voltaic cell Big Chemical Encyclopedia Zinc And Copper Galvanic Cell To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction. Zinc And Copper Galvanic Cell.
From stock.adobe.com
Illustration of galvanic cell consists of zinc and copper. Stock Vector Adobe Stock Zinc And Copper Galvanic Cell From the information given in. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. A simple electrochemical cell can be made from copper and zinc metals. Zinc And Copper Galvanic Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Zinc And Copper Galvanic Cell A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. From the information given in. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of. Zinc And Copper Galvanic Cell.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Zinc And Copper Galvanic Cell In the process of the reaction,. From the information given in. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. To illustrate the basic principles of a galvanic cell, let’s. Zinc And Copper Galvanic Cell.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID235007 Zinc And Copper Galvanic Cell To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. In the process of the reaction,. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc. Zinc And Copper Galvanic Cell.
From stock.adobe.com
Electrochemical cell diagram. Galvanic cell or voltaic cell. Zinc anode and copper cathode Zinc And Copper Galvanic Cell In the process of the reaction,. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc. Zinc And Copper Galvanic Cell.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Zinc And Copper Galvanic Cell Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. From the information given in. In the process of the reaction,. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. To illustrate the. Zinc And Copper Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Zinc And Copper Galvanic Cell Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2. Zinc And Copper Galvanic Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation ID1195562 Zinc And Copper Galvanic Cell One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. From the information given in. In the process of the reaction,. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic. Zinc And Copper Galvanic Cell.
From cider.uoregon.edu
Electrochemcial Cell Demonstration Voltaic Cell Zinc/Copper CIDER Zinc And Copper Galvanic Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield. Zinc And Copper Galvanic Cell.
From quizlet.com
Label the zinc/copper galvanic cell Diagram Quizlet Zinc And Copper Galvanic Cell One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates.. Zinc And Copper Galvanic Cell.
From 2012books.lardbucket.org
Describing Electrochemical Cells Zinc And Copper Galvanic Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. From the information given in. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. To illustrate. Zinc And Copper Galvanic Cell.
From www.youtube.com
How it works! Galvanic cell / Daniell cell / Copper zinc battery (3D Animation) YouTube Zinc And Copper Galvanic Cell In the process of the reaction,. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. From the information given in. Use cell notation to describe the. Zinc And Copper Galvanic Cell.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Zinc And Copper Galvanic Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. In the process of the reaction,. From the information given in. A simple electrochemical. Zinc And Copper Galvanic Cell.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary Zinc And Copper Galvanic Cell To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates.. Zinc And Copper Galvanic Cell.
From thegoodchemistguide.blogspot.com
The Good Chemist's Guide Copper Zinc Galvanic Cell Zinc And Copper Galvanic Cell One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. In the process of the reaction,. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is. Zinc And Copper Galvanic Cell.
From mavink.com
Zinc Copper Galvanic Cell Zinc And Copper Galvanic Cell In the process of the reaction,. From the information given in. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. Galvanic cell with zinc and copper depicts a voltaic cell and shows how. Zinc And Copper Galvanic Cell.
From www.researchgate.net
(PDF) Better Batteries Through Electrochemistry Zinc And Copper Galvanic Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. From the information given in. In the process of the reaction,. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A simple electrochemical cell can be made from copper and zinc metals with. Zinc And Copper Galvanic Cell.
From tecnico.aspillagahornauer.cl
Electrochemical Cells, 41 OFF Zinc And Copper Galvanic Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. Galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric current. A simple. Zinc And Copper Galvanic Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2810901 Zinc And Copper Galvanic Cell A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons.. Zinc And Copper Galvanic Cell.