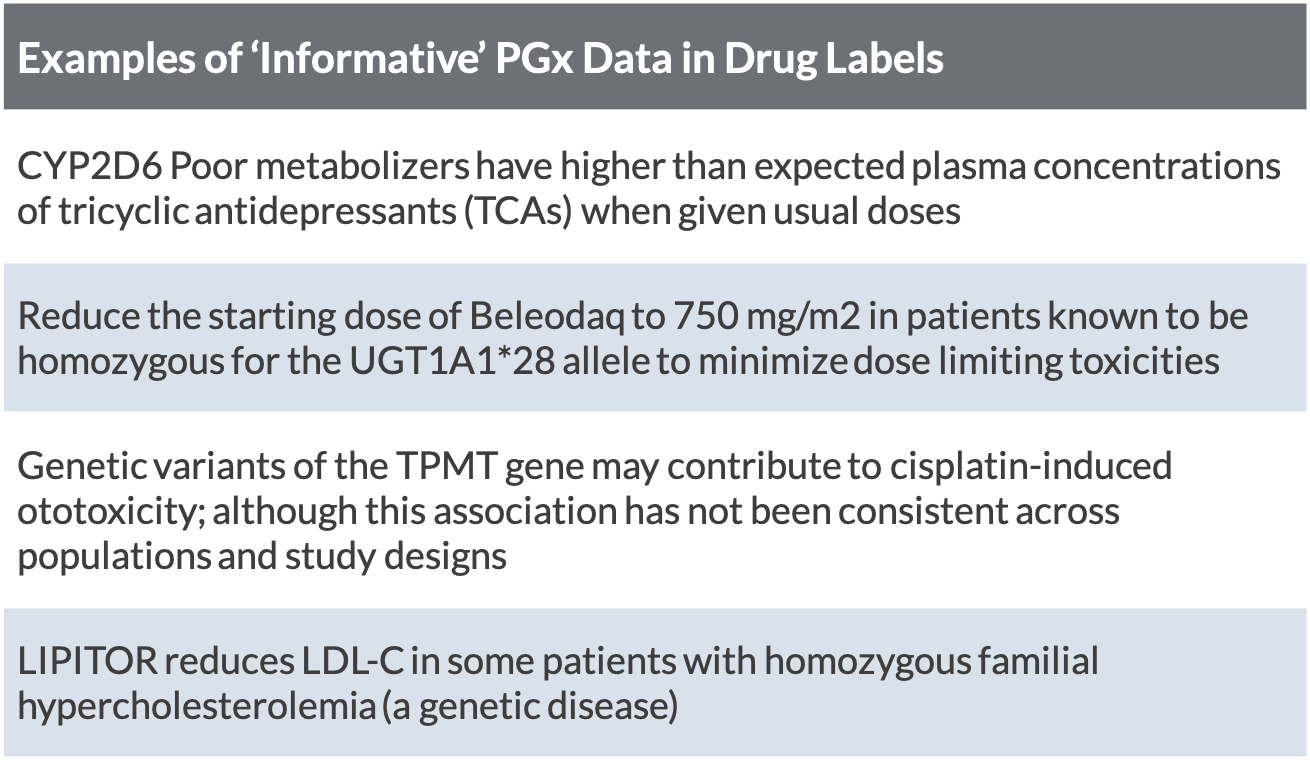
Fda Medication Labeling Requirements . Instructions for use, not all. What are the formats for the prescribing. Proposed by the drug company, reviewed by the fda, and. Medication guides, patient package inserts, and. When should prescribing information be updated? (1) the labeling must contain a summary of the. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: What is the prescribing information? For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly.
from animalia-life.club
Instructions for use, not all. When should prescribing information be updated? Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Proposed by the drug company, reviewed by the fda, and. Medication guides, patient package inserts, and. What are the formats for the prescribing. What is the prescribing information? (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. (1) the labeling must contain a summary of the.
Fda Drug Labeling Requirements
Fda Medication Labeling Requirements For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. What are the formats for the prescribing. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. What is the prescribing information? (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. (1) the labeling must contain a summary of the. Instructions for use, not all. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Medication guides, patient package inserts, and. Proposed by the drug company, reviewed by the fda, and. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. When should prescribing information be updated?
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Medication Labeling Requirements (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Instructions for use, not all. What are the formats for the prescribing. (1) the labeling must contain a summary of the. What is the prescribing information? When should prescribing information be updated? Proposed by the drug company, reviewed by. Fda Medication Labeling Requirements.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Medication Labeling Requirements For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. Proposed by the drug company, reviewed by the fda, and. Medication guides, patient package inserts, and. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Instructions for use, not all. What. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements Proposed by the drug company, reviewed by the fda, and. Medication guides, patient package inserts, and. (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements Instructions for use, not all. Medication guides, patient package inserts, and. When should prescribing information be updated? What is the prescribing information? (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe. Fda Medication Labeling Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Fda Medication Labeling Requirements When should prescribing information be updated? What are the formats for the prescribing. Instructions for use, not all. Proposed by the drug company, reviewed by the fda, and. (1) the labeling must contain a summary of the. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. For more information on. Fda Medication Labeling Requirements.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Fda Medication Labeling Requirements What are the formats for the prescribing. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. What is the prescribing information? (1) the labeling must. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements Instructions for use, not all. What is the prescribing information? (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (a) this part sets forth requirements. Fda Medication Labeling Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Medication Labeling Requirements (1) the labeling must contain a summary of the. When should prescribing information be updated? (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food. Fda Medication Labeling Requirements.
From www.fda.gov
OTC Drug Facts Label FDA Fda Medication Labeling Requirements Medication guides, patient package inserts, and. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. Proposed by the drug company, reviewed by the fda, and. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Instructions for use, not all. (a). Fda Medication Labeling Requirements.
From fyozknlqk.blob.core.windows.net
How To Read A Prescription Label at Steven Garcia blog Fda Medication Labeling Requirements When should prescribing information be updated? (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. What is the prescribing information? (1) the labeling must contain a summary of the. Medication guides, patient package inserts, and. (a) the appropriate fda center director may grant an exception or alternative to. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements Instructions for use, not all. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. Medication guides, patient package inserts, and. Proposed by the drug company, reviewed by the fda, and.. Fda Medication Labeling Requirements.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Fda Medication Labeling Requirements What are the formats for the prescribing. What is the prescribing information? For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Instructions for use, not all. When should prescribing. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (1) the labeling must contain a summary of the. Instructions for use, not all. Prescription drug. Fda Medication Labeling Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Fda Medication Labeling Requirements Medication guides, patient package inserts, and. (1) the labeling must contain a summary of the. (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. Proposed by the drug company, reviewed by the fda, and. (a) this part sets forth requirements for patient labeling for. Fda Medication Labeling Requirements.
From www.i3cglobal.com
FDA Labelling Requirements Guidance and Pricing Fda Medication Labeling Requirements For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. Proposed by the drug company, reviewed by the fda, and. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Medication guides, patient package inserts, and. What is the prescribing information? (a) the appropriate fda center director. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements Proposed by the drug company, reviewed by the fda, and. What is the prescribing information? Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. Medication. Fda Medication Labeling Requirements.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Medication Labeling Requirements For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. What is the prescribing information? Proposed by the drug company, reviewed by the fda, and. Medication guides, patient package inserts, and. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food. Fda Medication Labeling Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Fda Medication Labeling Requirements What is the prescribing information? Instructions for use, not all. When should prescribing information be updated? (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. (1) the labeling must contain a summary of the. For more information on labeling, including physician labeling rule (plr). Fda Medication Labeling Requirements.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Fda Medication Labeling Requirements (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Medication guides, patient package inserts, and. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and.. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements (1) the labeling must contain a summary of the. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. When should prescribing information be updated? Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Medication guides, patient package inserts, and. What. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements (1) the labeling must contain a summary of the. Instructions for use, not all. What is the prescribing information? When should prescribing information be updated? Medication guides, patient package inserts, and. (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. For more information on. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Proposed by the drug company, reviewed by the fda, and. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Instructions for use, not all. (a) the appropriate fda center. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements When should prescribing information be updated? (1) the labeling must contain a summary of the. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Medication guides, patient package inserts, and. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. Proposed. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements What are the formats for the prescribing. Instructions for use, not all. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Proposed by the drug company, reviewed by the fda, and. Medication guides, patient package inserts, and. When should prescribing information be updated? (a) this part sets forth requirements for patient labeling for human prescription. Fda Medication Labeling Requirements.
From www.fda.gov
Patient Labeling Resources FDA Fda Medication Labeling Requirements (1) the labeling must contain a summary of the. When should prescribing information be updated? Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. Human prescription drug labeling (1) contains a summary of the essential scientific. Fda Medication Labeling Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Medication Labeling Requirements (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. When should prescribing information be updated? What are the formats for the prescribing. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Medication guides, patient package inserts, and. Human prescription. Fda Medication Labeling Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Medication Labeling Requirements (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Proposed by the drug company, reviewed by the fda, and. What is the prescribing information? For more information on labeling, including. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements What are the formats for the prescribing. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. What is the prescribing information? Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (a) this part sets forth requirements for patient labeling for. Fda Medication Labeling Requirements.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Fda Medication Labeling Requirements Proposed by the drug company, reviewed by the fda, and. (1) the labeling must contain a summary of the. (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. Instructions for use, not all. For more information on labeling, including physician labeling rule (plr) requirements,. Fda Medication Labeling Requirements.
From www.fda.gov.ph
Draft for Comments Guidelines on Labeling Requirements of Drug Fda Medication Labeling Requirements Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: What are the formats for the prescribing. Proposed by the drug company, reviewed by the fda, and. What is the prescribing information? When should prescribing information be updated? For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools,. Fda Medication Labeling Requirements.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Fda Medication Labeling Requirements What are the formats for the prescribing. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. When should prescribing information be updated? Proposed by the drug company, reviewed by the fda, and. What is the prescribing information? (a) this part sets forth requirements for patient labeling for human prescription drug. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. Instructions for use, not all. Medication guides, patient package inserts, and. (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly. When should prescribing information be. Fda Medication Labeling Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Medication Labeling Requirements When should prescribing information be updated? Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample templates and format tools, and. (a) the appropriate fda center director may grant an exception or alternative to any provision listed. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements Medication guides, patient package inserts, and. What is the prescribing information? (1) the labeling must contain a summary of the. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Proposed by the drug company,. Fda Medication Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Labeling Requirements What is the prescribing information? Instructions for use, not all. Medication guides, patient package inserts, and. When should prescribing information be updated? Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (a) the appropriate fda center director may grant an exception or alternative to any provision listed in paragraph (f). Fda Medication Labeling Requirements.