Medical Device Instructions For Use Guidelines . Instructions for use (ifu) is: Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. Form of prescription drug labeling. Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Instructions for use are generally defined as the information a medical. Regulation 2021/2226 on electronic instructions for. Most of the requirements for the instructions for use can be found in annex i, section 20. The seven top things to consider when writing instructions for use for medical devices are: What is an ifu and what is an eifu (electronic instructions for use)?
from ar.inspiredpencil.com
The seven top things to consider when writing instructions for use for medical devices are: Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. What is an ifu and what is an eifu (electronic instructions for use)? Instructions for use are generally defined as the information a medical. Regulation 2021/2226 on electronic instructions for. Instructions for use (ifu) is: Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Most of the requirements for the instructions for use can be found in annex i, section 20. Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. Form of prescription drug labeling.
Life Instructions
Medical Device Instructions For Use Guidelines Regulation 2021/2226 on electronic instructions for. Instructions for use (ifu) is: Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Regulation 2021/2226 on electronic instructions for. Most of the requirements for the instructions for use can be found in annex i, section 20. What is an ifu and what is an eifu (electronic instructions for use)? Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. Form of prescription drug labeling. Instructions for use are generally defined as the information a medical. Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. The seven top things to consider when writing instructions for use for medical devices are:
From pdihc.com
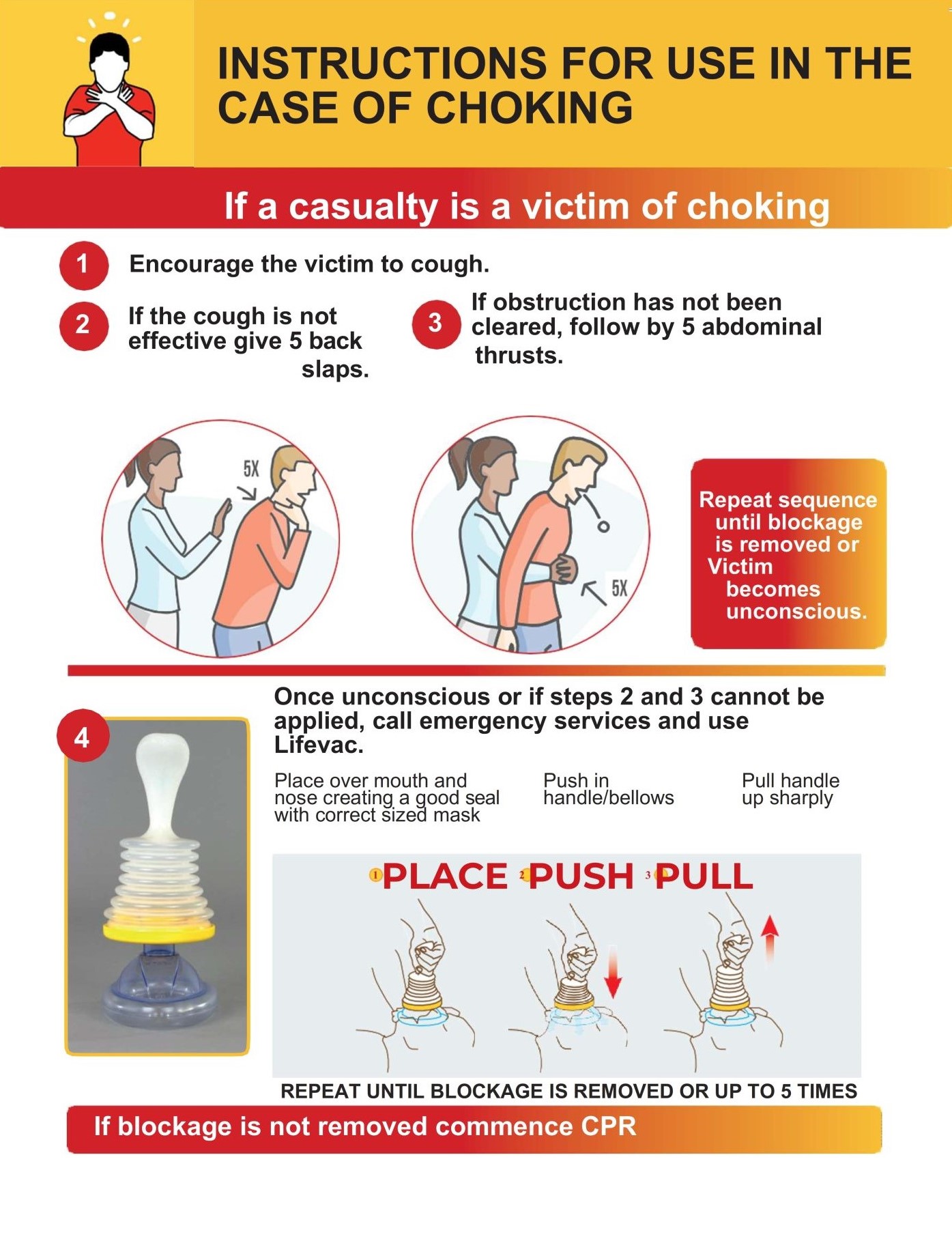
Prevantics® Device Swab Instructions For Use (IFU) Sign PDI Healthcare Medical Device Instructions For Use Guidelines Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. Regulation 2021/2226 on electronic instructions for. What is an ifu and what is an eifu (electronic instructions for use)? Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. Process for medical. Medical Device Instructions For Use Guidelines.
From blog.sierralabs.com
A Simple Guide to 510(k) Applications for Medical Devices Medical Device Instructions For Use Guidelines Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. What is an ifu and what is an eifu (electronic instructions for use)? Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. Instructions for use (ifu) is: Instructions for use are. Medical Device Instructions For Use Guidelines.
From www.team-consulting.com
Medical Device Instructions for Use Team Consulting Medical Device Instructions For Use Guidelines Regulation 2021/2226 on electronic instructions for. Instructions for use are generally defined as the information a medical. Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Most of the requirements for the instructions for use can be found in annex i, section 20. Instructions for use (ifu) is: Ensuring healthcare. Medical Device Instructions For Use Guidelines.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device Instructions For Use Guidelines Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. Regulation 2021/2226 on. Medical Device Instructions For Use Guidelines.
From instrktiv.com
IFU Medical Device Create Instructions For Use Medical Device Instructions For Use Guidelines Most of the requirements for the instructions for use can be found in annex i, section 20. Instructions for use are generally defined as the information a medical. Instructions for use (ifu) is: Regulation 2021/2226 on electronic instructions for. Form of prescription drug labeling. What is an ifu and what is an eifu (electronic instructions for use)? The seven top. Medical Device Instructions For Use Guidelines.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Instructions For Use Guidelines Regulation 2021/2226 on electronic instructions for. The seven top things to consider when writing instructions for use for medical devices are: Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. Ensuring healthcare. Medical Device Instructions For Use Guidelines.
From unionmedico.com
Instructions for use Union Medico Medical Device Instructions For Use Guidelines Most of the requirements for the instructions for use can be found in annex i, section 20. Instructions for use (ifu) is: Form of prescription drug labeling. Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. What is an ifu and what is an eifu (electronic instructions for use)?. Medical Device Instructions For Use Guidelines.
From usermanual.wiki
Aulisa Medical Devices Technologies GA1001 Bluetooth 4.0 BTLE Sensor Medical Device Instructions For Use Guidelines Regulation 2021/2226 on electronic instructions for. Most of the requirements for the instructions for use can be found in annex i, section 20. Instructions for use are generally defined as the information a medical. What is an ifu and what is an eifu (electronic instructions for use)? Process for medical devices, including providing strict guidelines for labeling and the inclusion. Medical Device Instructions For Use Guidelines.
From studylib.net
instructions for use of the device dkf580 medical Medical Device Instructions For Use Guidelines Instructions for use are generally defined as the information a medical. Regulation 2021/2226 on electronic instructions for. What is an ifu and what is an eifu (electronic instructions for use)? Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. Process for medical devices, including providing strict guidelines for labeling and the. Medical Device Instructions For Use Guidelines.
From medicalxpress.com
Revamped guidance 'cleans up' medical device instructions for use Medical Device Instructions For Use Guidelines Regulation 2021/2226 on electronic instructions for. Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. Instructions for use (ifu) is: Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. The seven top things to consider when writing instructions for use. Medical Device Instructions For Use Guidelines.
From www.blackstonemedicalservices.com
Instructions Blackstone Medical Services Medical Device Instructions For Use Guidelines The seven top things to consider when writing instructions for use for medical devices are: Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Regulation 2021/2226 on electronic instructions for. Form of prescription drug labeling. What is an ifu and what is an eifu (electronic instructions for use)? Most of. Medical Device Instructions For Use Guidelines.
From cektzqdq.blob.core.windows.net
Medical Device Regulations In Australia at Martha Murray blog Medical Device Instructions For Use Guidelines Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Form of prescription drug labeling. The seven top things to consider when writing instructions for use for medical devices are: Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. What. Medical Device Instructions For Use Guidelines.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Instructions For Use Guidelines Regulation 2021/2226 on electronic instructions for. Form of prescription drug labeling. Most of the requirements for the instructions for use can be found in annex i, section 20. Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. The seven top things to consider when writing instructions for use for medical. Medical Device Instructions For Use Guidelines.
From www.cirs-group.com
Administration Regulations for Instruction and Label of Medical Device Medical Device Instructions For Use Guidelines Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. Regulation 2021/2226 on electronic instructions for. Instructions for use are generally defined as the information a medical. Most of the requirements for the instructions for use can be found in annex i, section 20. Process for medical devices, including providing. Medical Device Instructions For Use Guidelines.
From www.regdesk.co
FDA Guidance on Reprocessing Medical Devices Criteria 13 for Medical Device Instructions For Use Guidelines Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. What is an ifu and what is an eifu (electronic instructions for use)? The seven top things to consider when writing instructions for use for medical devices are: Instructions for use (ifu) is: Form of prescription drug labeling. Regulation 2021/2226 on electronic. Medical Device Instructions For Use Guidelines.
From manualzz.com
Instructions for Use Manualzz Medical Device Instructions For Use Guidelines Instructions for use (ifu) is: What is an ifu and what is an eifu (electronic instructions for use)? Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. The seven top. Medical Device Instructions For Use Guidelines.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Medical Device Instructions For Use Guidelines Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Form of prescription drug labeling. What is an ifu and what is an eifu (electronic instructions for use)? Most of the. Medical Device Instructions For Use Guidelines.
From medicaldevicehq.com
How to write instructions for use for medical devices Medical Device HQ Medical Device Instructions For Use Guidelines Most of the requirements for the instructions for use can be found in annex i, section 20. Instructions for use (ifu) is: Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Regulation 2021/2226 on electronic instructions for. Form of prescription drug labeling. Labelling1 serves to identify a device and its. Medical Device Instructions For Use Guidelines.
From www.sampleformats.org
Instruction Manual Templates 10+ Free Printable Word & PDF Formats Medical Device Instructions For Use Guidelines Instructions for use (ifu) is: Instructions for use are generally defined as the information a medical. Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. What is an ifu and what is an eifu (electronic instructions for use)? The seven top things to consider when writing instructions for use. Medical Device Instructions For Use Guidelines.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Instructions For Use Guidelines Instructions for use (ifu) is: Regulation 2021/2226 on electronic instructions for. Form of prescription drug labeling. The seven top things to consider when writing instructions for use for medical devices are: Most of the requirements for the instructions for use can be found in annex i, section 20. Ensuring healthcare providers and patients understand and use medical device products safely. Medical Device Instructions For Use Guidelines.
From www.stc.org
Developing Medical Device Instructions for Use to Facilitate Learning Medical Device Instructions For Use Guidelines Instructions for use are generally defined as the information a medical. Form of prescription drug labeling. Regulation 2021/2226 on electronic instructions for. Instructions for use (ifu) is: Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. Most of the requirements for the instructions for use can be found in annex i,. Medical Device Instructions For Use Guidelines.
From templatelab.com
40 Free Instruction Manual Templates [Operation / User Manual] Medical Device Instructions For Use Guidelines Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Most of the requirements for the instructions for use can be found in annex i, section 20. Instructions for use are generally defined as the information a medical. Labelling1 serves to identify a device and its manufacturer, and to communicate information. Medical Device Instructions For Use Guidelines.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Instructions For Use Guidelines Instructions for use are generally defined as the information a medical. Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. Regulation 2021/2226 on electronic instructions for. Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Instructions for use (ifu). Medical Device Instructions For Use Guidelines.
From www.team-consulting.com
IFU medical device experts Team Consulting Medical Device Instructions For Use Guidelines Most of the requirements for the instructions for use can be found in annex i, section 20. The seven top things to consider when writing instructions for use for medical devices are: Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Labelling1 serves to identify a device and its manufacturer,. Medical Device Instructions For Use Guidelines.
From medicaldevicehq.com
How to write instructions for use for medical devices Medical Device HQ Medical Device Instructions For Use Guidelines Instructions for use (ifu) is: Instructions for use are generally defined as the information a medical. Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. Regulation 2021/2226 on electronic instructions for. The. Medical Device Instructions For Use Guidelines.
From revul.com.ua
Instructions for use of medical device absorbent hemostatic preparation Medical Device Instructions For Use Guidelines Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. Form of prescription drug labeling. Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. The seven top things to consider when writing instructions for use for medical devices are: Instructions for use. Medical Device Instructions For Use Guidelines.
From www.stc.org
Developing Medical Device Instructions for Use to Facilitate Learning Medical Device Instructions For Use Guidelines The seven top things to consider when writing instructions for use for medical devices are: Most of the requirements for the instructions for use can be found in annex i, section 20. What is an ifu and what is an eifu (electronic instructions for use)? Instructions for use (ifu) is: Regulation 2021/2226 on electronic instructions for. Form of prescription drug. Medical Device Instructions For Use Guidelines.
From omcmedical.com
Electronic Instructions for use Of Medical Devices OMC Medical Medical Device Instructions For Use Guidelines Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. Instructions for use (ifu) is: The seven top things to consider when writing instructions for use for medical devices are: Regulation 2021/2226 on electronic instructions for. Form of prescription drug labeling. What is an ifu and what is an eifu. Medical Device Instructions For Use Guidelines.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Medical Device Instructions For Use Guidelines Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Most of the requirements for the instructions for use can be found in annex i, section 20. Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. Regulation 2021/2226 on electronic instructions for.. Medical Device Instructions For Use Guidelines.
From www.asthmafoundation.org.nz
Inhaler Devices Identification Chart Asthma Foundation NZ Medical Device Instructions For Use Guidelines The seven top things to consider when writing instructions for use for medical devices are: Labelling1 serves to identify a device and its manufacturer, and to communicate information on safety, use and performance. Most of the requirements for the instructions for use can be found in annex i, section 20. Instructions for use are generally defined as the information a. Medical Device Instructions For Use Guidelines.
From fr.slideshare.net
Medical Device FDA Regulations and Classifications infographic Medical Device Instructions For Use Guidelines Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. The seven top things to consider when writing instructions for use for medical devices are: Most of the requirements for the instructions for use can be found in annex i, section 20. What is an ifu and what is an eifu. Medical Device Instructions For Use Guidelines.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Instructions For Use Guidelines Instructions for use (ifu) is: Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. What is an ifu and what is an eifu (electronic instructions for use)? The seven top things to consider when writing instructions for use for medical devices are: Process for medical devices, including providing strict. Medical Device Instructions For Use Guidelines.
From ar.inspiredpencil.com
Life Instructions Medical Device Instructions For Use Guidelines Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. Form of prescription drug labeling. The seven top things to consider when writing instructions for use for medical devices are: Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Instructions. Medical Device Instructions For Use Guidelines.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Instructions For Use Guidelines The seven top things to consider when writing instructions for use for medical devices are: Instructions for use are generally defined as the information a medical. Process for medical devices, including providing strict guidelines for labeling and the inclusion of instructions for the device user. Ensuring healthcare providers and patients understand and use medical device products safely and effectively is. Medical Device Instructions For Use Guidelines.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Instructions For Use Guidelines What is an ifu and what is an eifu (electronic instructions for use)? Form of prescription drug labeling. Most of the requirements for the instructions for use can be found in annex i, section 20. Ensuring healthcare providers and patients understand and use medical device products safely and effectively is a focus of the eu. Instructions for use (ifu) is:. Medical Device Instructions For Use Guidelines.