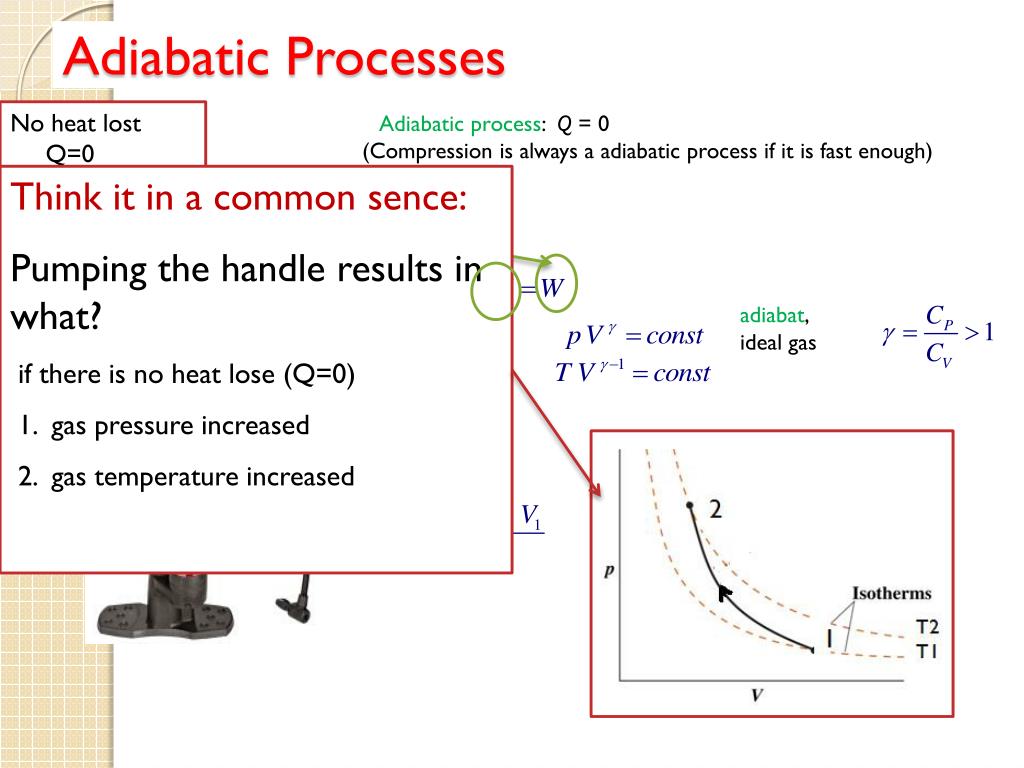
Are All Reversible Processes Adiabatic . The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. An adiabatic process is a reversible process with constant entropy for an ideal gas. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and for an adiabatic process, we have. Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. The change in entropy for a system undergoing an adiabatic path is zero. A reversible process is one in which both the. For a system that undergoes an adiabatic irreversible process, you need to.
from www.slideserve.com
An adiabatic process is a reversible process with constant entropy for an ideal gas. The change in entropy for a system undergoing an adiabatic path is zero. Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and for an adiabatic process, we have. A reversible process is one in which both the. For a system that undergoes an adiabatic irreversible process, you need to. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle.
PPT THERMODYNAMIC an introduction PowerPoint Presentation, free
Are All Reversible Processes Adiabatic Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. A reversible process is one in which both the. For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and for an adiabatic process, we have. An adiabatic process is a reversible process with constant entropy for an ideal gas. The change in entropy for a system undergoing an adiabatic path is zero. Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. For a system that undergoes an adiabatic irreversible process, you need to.
From narodnatribuna.info
What Is Adiabatic Process Explanation Mechanical Booster Are All Reversible Processes Adiabatic An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. A reversible process is one in which both the. For a system that undergoes an adiabatic irreversible process, you need to. For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align}. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT THERMODYNAMIC an introduction PowerPoint Presentation, free Are All Reversible Processes Adiabatic An adiabatic process is a reversible process with constant entropy for an ideal gas. A reversible process is one in which both the. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. The adiabatic process is a thermodynamic process in which there is no. Are All Reversible Processes Adiabatic.
From www.youtube.com
Thermodynamic Processes Isobaric, Isochoric, Isothermal and Adiabatic Are All Reversible Processes Adiabatic Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. The adiabatic process is a thermodynamic process in which there is no heat transfer. Are All Reversible Processes Adiabatic.
From www.tf.uni-kiel.de
Adiabatic changes Poisson equations Are All Reversible Processes Adiabatic For a system that undergoes an adiabatic irreversible process, you need to. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. A reversible process is one in which both the. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a. Are All Reversible Processes Adiabatic.
From www.studocu.com
Isentropic or Reversible Adiabatic Process Adiabatic simply means no Are All Reversible Processes Adiabatic Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. An adiabatic process is a reversible process with constant entropy for an ideal gas. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes.. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Are All Reversible Processes Adiabatic The change in entropy for a system undergoing an adiabatic path is zero. Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. For a system that undergoes an adiabatic irreversible process, you need to. For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and for an adiabatic process, we. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Reversible Process PowerPoint Presentation, free download ID527789 Are All Reversible Processes Adiabatic The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. For a system that undergoes an adiabatic irreversible process, you need to. The change in entropy for a system undergoing an adiabatic path is zero. Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in. Are All Reversible Processes Adiabatic.
From appliancesleader.blogspot.com
25 Reversible Adiabatic Process AppliancesLeader Are All Reversible Processes Adiabatic An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and for an adiabatic process, we have. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Adiabatic Processes PowerPoint Presentation, free download ID Are All Reversible Processes Adiabatic The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. A reversible process is one in which both the. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. For a reversible process, we. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Adiabatic Processes PowerPoint Presentation, free download ID Are All Reversible Processes Adiabatic A reversible process is one in which both the. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. An adiabatic process is a reversible process with constant entropy for an ideal gas. For a reversible process, we have the relation \begin{align} ds = \frac{\delta. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Relation between C P & C V PowerPoint Presentation, free download Are All Reversible Processes Adiabatic The change in entropy for a system undergoing an adiabatic path is zero. Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. For a system that undergoes an adiabatic irreversible process, you need to. A reversible process is one in which both the. Both isothermal and adiabatic processes sketched on a pv graph (discussed. Are All Reversible Processes Adiabatic.
From adpokat.github.io
What Is Adiabatic Process Are All Reversible Processes Adiabatic The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. An adiabatic process is a reversible process with constant entropy for an ideal gas. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes.. Are All Reversible Processes Adiabatic.
From www.studocu.com
Isentropic or Reversible Adiabatic Process Adiabatic simply means no Are All Reversible Processes Adiabatic The change in entropy for a system undergoing an adiabatic path is zero. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. A reversible process is one in which both the. An adiabatic. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Short Version 18. Heat, Work, & First Law of Thermodynamics Are All Reversible Processes Adiabatic For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and for an adiabatic process, we have. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. For a system that undergoes an adiabatic irreversible process, you need to. The change. Are All Reversible Processes Adiabatic.
From www.youtube.com
Adiabatic Reversible Process For Ideal Gas YouTube Are All Reversible Processes Adiabatic An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align}. Are All Reversible Processes Adiabatic.
From www.youtube.com
Relation between Pressure, Volume and Temperature in Adiabatic Process Are All Reversible Processes Adiabatic The change in entropy for a system undergoing an adiabatic path is zero. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and for an adiabatic process, we have. Both. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Are All Reversible Processes Adiabatic An adiabatic process is a reversible process with constant entropy for an ideal gas. Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. The change in entropy for a. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Are All Reversible Processes Adiabatic An adiabatic process is a reversible process with constant entropy for an ideal gas. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. A reversible process is one in which both the. For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and for. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Adiabatic Processes PowerPoint Presentation, free download ID Are All Reversible Processes Adiabatic A reversible process is one in which both the. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. For a system that undergoes. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Are All Reversible Processes Adiabatic An adiabatic process is a reversible process with constant entropy for an ideal gas. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. A reversible process is one in which both the. The change in entropy for a system undergoing an adiabatic path is zero. For a reversible. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free Are All Reversible Processes Adiabatic The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. An adiabatic process is a reversible process with constant entropy for an ideal gas. A reversible process is one in which both the. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Are All Reversible Processes Adiabatic An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the. Are All Reversible Processes Adiabatic.
From www.youtube.com
Reversible Adiabatic process Thermodynamics Tricks by Komali mam Are All Reversible Processes Adiabatic For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and for an adiabatic process, we have. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. For a system that undergoes an adiabatic irreversible process, you need to. The change in entropy for a. Are All Reversible Processes Adiabatic.
From learncheme.com
LearnChemE Are All Reversible Processes Adiabatic For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and for an adiabatic process, we have. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Are All Reversible Processes Adiabatic Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. An adiabatic process. Are All Reversible Processes Adiabatic.
From monomole.com
Reversible adiabatic processes Mono Mole Are All Reversible Processes Adiabatic For a system that undergoes an adiabatic irreversible process, you need to. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. The change in entropy for a system undergoing an adiabatic path is zero. Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in. Are All Reversible Processes Adiabatic.
From senocone.blogspot.com
Adiabatic Process Ppt Seno Are All Reversible Processes Adiabatic Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. The change in entropy for a system undergoing an adiabatic path is zero. For a system that undergoes an adiabatic irreversible process, you need to. A reversible process is one in which both the. For a reversible process, we have the relation \begin{align} ds =. Are All Reversible Processes Adiabatic.
From www.chemistrylearner.com
Adiabatic Process Definition, Examples, and Equations Are All Reversible Processes Adiabatic For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and for an adiabatic process, we have. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. A reversible process is one in which both the. An adiabatic process is a reversible process with constant. Are All Reversible Processes Adiabatic.
From www.toppr.com
Work done in reversible adiabatic process by an ideal gas is given by Are All Reversible Processes Adiabatic The change in entropy for a system undergoing an adiabatic path is zero. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. A reversible process is one in which both the. An adiabatic process is a reversible process with constant entropy for an ideal gas. For a reversible. Are All Reversible Processes Adiabatic.
From www.slideserve.com
PPT Adiabatic Reversible Process q=0 Ideal gas PowerPoint Are All Reversible Processes Adiabatic An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. A reversible process is one in which both the. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. Both isothermal and adiabatic processes. Are All Reversible Processes Adiabatic.
From 88guru.com
Adiabatic Process Definition, Equation, Reversible 88Guru Are All Reversible Processes Adiabatic Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. A reversible process is one in which both the. For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and. Are All Reversible Processes Adiabatic.
From 88guru.com
Adiabatic Process Definition, Equation, Reversible 88Guru Are All Reversible Processes Adiabatic A reversible process is one in which both the. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. The change in entropy for a system undergoing an. Are All Reversible Processes Adiabatic.
From slideplayer.com
Dr.Salwa Al Saleh Work and Heat Lecture ppt download Are All Reversible Processes Adiabatic Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. An adiabatic process is one in which no heat enters or leaves the system, and hence, for a reversible adiabatic process the first law takes. The adiabatic process is a thermodynamic process in which there is no heat transfer. Are All Reversible Processes Adiabatic.
From www.mechanicalbooster.com
What is Adiabatic Process Explanation Mechanical Booster Are All Reversible Processes Adiabatic The change in entropy for a system undergoing an adiabatic path is zero. For a reversible process, we have the relation \begin{align} ds = \frac{\delta q}{t} \end{align} and for an adiabatic process, we have. A reversible process is one in which both the. For a system that undergoes an adiabatic irreversible process, you need to. An adiabatic process is a. Are All Reversible Processes Adiabatic.
From en.wikipedia.org
Adiabatic process Wikipedia Are All Reversible Processes Adiabatic The change in entropy for a system undergoing an adiabatic path is zero. Both isothermal and adiabatic processes such as shown in figure 15.13 are reversible in principle. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. For a system that undergoes an adiabatic irreversible process, you need. Are All Reversible Processes Adiabatic.