Does Water Temperature Affect Pressure . early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. in this section we will look at how the pressure and temperature effect solubility. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. The following figure illustrates the molecular behavior of a. When we look at pressure effects, we are considering the. pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. Liquid water properties at temperatures between melting point and boiling point. yes, at constant density, the pressure increases as the temperature does:
from chart-studio.plotly.com
Liquid water properties at temperatures between melting point and boiling point. The following figure illustrates the molecular behavior of a. When we look at pressure effects, we are considering the. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. in this section we will look at how the pressure and temperature effect solubility. pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. yes, at constant density, the pressure increases as the temperature does:
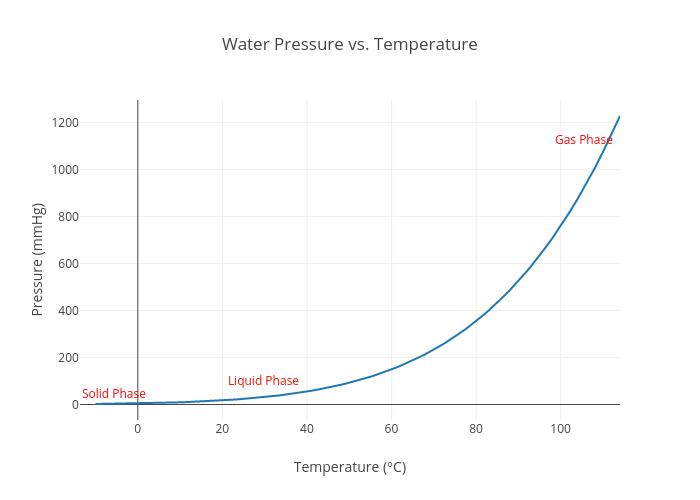
Water Pressure vs. Temperature line chart made by 18youngt plotly
Does Water Temperature Affect Pressure in this section we will look at how the pressure and temperature effect solubility. pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. When we look at pressure effects, we are considering the. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. yes, at constant density, the pressure increases as the temperature does: The following figure illustrates the molecular behavior of a. Liquid water properties at temperatures between melting point and boiling point. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. in this section we will look at how the pressure and temperature effect solubility.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does Water Temperature Affect Pressure yes, at constant density, the pressure increases as the temperature does: pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. early scientists. Does Water Temperature Affect Pressure.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Does Water Temperature Affect Pressure When we look at pressure effects, we are considering the. lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. The following figure illustrates the molecular behavior of a. pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any. Does Water Temperature Affect Pressure.
From www.slideserve.com
PPT 22 Properties of water PowerPoint Presentation ID379006 Does Water Temperature Affect Pressure lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of. Does Water Temperature Affect Pressure.
From dxoboaesr.blob.core.windows.net
Why Does Temp Increase With Pressure at Shane Bailey blog Does Water Temperature Affect Pressure pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. When we look at pressure effects, we are considering the. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding.. Does Water Temperature Affect Pressure.
From quizdbpharmacies.z4.web.core.windows.net
How Does Temperature Affect Matter Does Water Temperature Affect Pressure in this section we will look at how the pressure and temperature effect solubility. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. Liquid water properties at temperatures between melting point and boiling point. early scientists explored the relationships among the pressure of a gas (p) and. Does Water Temperature Affect Pressure.
From dxoboaesr.blob.core.windows.net
Why Does Temp Increase With Pressure at Shane Bailey blog Does Water Temperature Affect Pressure in this section we will look at how the pressure and temperature effect solubility. The following figure illustrates the molecular behavior of a. pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. lets say we have a tank with a fixed. Does Water Temperature Affect Pressure.
From dxozivmnt.blob.core.windows.net
How Does Pressure Affect Water at Dean Morton blog Does Water Temperature Affect Pressure in this section we will look at how the pressure and temperature effect solubility. When we look at pressure effects, we are considering the. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. yes, at constant density, the pressure increases as the temperature does: lets say. Does Water Temperature Affect Pressure.
From www.slideserve.com
PPT Fluid Mechanics PowerPoint Presentation, free download ID6711078 Does Water Temperature Affect Pressure The following figure illustrates the molecular behavior of a. Liquid water properties at temperatures between melting point and boiling point. When we look at pressure effects, we are considering the. lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. yes, at constant density, the pressure increases as the temperature. Does Water Temperature Affect Pressure.
From chart-studio.plotly.com
Water Pressure vs. Temperature line chart made by 18youngt plotly Does Water Temperature Affect Pressure early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. in this section we will look at how the pressure and temperature effect solubility. . Does Water Temperature Affect Pressure.
From quizrecentring.z21.web.core.windows.net
How Temperature Affect Solubility Does Water Temperature Affect Pressure in this section we will look at how the pressure and temperature effect solubility. yes, at constant density, the pressure increases as the temperature does: the change in specific volume for a given change in temperature is not the same at various beginning temperatures. The following figure illustrates the molecular behavior of a. pressure and temperature. Does Water Temperature Affect Pressure.
From www.researchgate.net
Relationship between temperature and surface tension of liquid water Does Water Temperature Affect Pressure yes, at constant density, the pressure increases as the temperature does: pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. the change. Does Water Temperature Affect Pressure.
From www.researchgate.net
Pressure±temperature phase diagram for water and average surface P±T Does Water Temperature Affect Pressure lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. When we look at pressure effects, we are considering the. the change in specific. Does Water Temperature Affect Pressure.
From www.researchgate.net
PressureTemperature diagram for water. Download Scientific Diagram Does Water Temperature Affect Pressure yes, at constant density, the pressure increases as the temperature does: When we look at pressure effects, we are considering the. lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. Liquid water properties at temperatures between melting point and boiling point. in this section we will look at. Does Water Temperature Affect Pressure.
From mungfali.com
Pressure Temperature Diagram Of Water Does Water Temperature Affect Pressure yes, at constant density, the pressure increases as the temperature does: early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. in this section we will look at how the pressure and temperature effect solubility. Liquid water properties at temperatures between melting point and. Does Water Temperature Affect Pressure.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Does Water Temperature Affect Pressure in this section we will look at how the pressure and temperature effect solubility. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. The following figure illustrates the molecular behavior of a. When we look at pressure effects, we are considering the. lets say we have a. Does Water Temperature Affect Pressure.
From pediaa.com
Relationship Between Pressure and Temperature Does Water Temperature Affect Pressure the change in specific volume for a given change in temperature is not the same at various beginning temperatures. When we look at pressure effects, we are considering the. in this section we will look at how the pressure and temperature effect solubility. Liquid water properties at temperatures between melting point and boiling point. pressure and temperature. Does Water Temperature Affect Pressure.
From www.researchgate.net
Relationship between temperature and pressure. Download Scientific Does Water Temperature Affect Pressure The following figure illustrates the molecular behavior of a. yes, at constant density, the pressure increases as the temperature does: the change in specific volume for a given change in temperature is not the same at various beginning temperatures. When we look at pressure effects, we are considering the. early scientists explored the relationships among the pressure. Does Water Temperature Affect Pressure.
From exorpxhjd.blob.core.windows.net
Evaporation Vapor Pressure Of Water at Marcus Quigley blog Does Water Temperature Affect Pressure in this section we will look at how the pressure and temperature effect solubility. pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v),. Does Water Temperature Affect Pressure.
From www.h2xengineering.com
Correlation Between Water Temperature and Friction Loss in Pipes Does Water Temperature Affect Pressure When we look at pressure effects, we are considering the. yes, at constant density, the pressure increases as the temperature does: early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. the change in specific volume for a given change in temperature is not. Does Water Temperature Affect Pressure.
From www.slideserve.com
PPT Atmospheric Dynamics PowerPoint Presentation, free download ID Does Water Temperature Affect Pressure lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. Liquid water properties at temperatures between melting point and boiling point. yes, at constant density, the pressure increases as the. Does Water Temperature Affect Pressure.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Does Water Temperature Affect Pressure lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. When we look at pressure effects, we are considering the. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. yes, at constant density, the pressure. Does Water Temperature Affect Pressure.
From mungfali.com
Water Pressure Temperature Phase Diagram Does Water Temperature Affect Pressure When we look at pressure effects, we are considering the. in this section we will look at how the pressure and temperature effect solubility. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. The following figure illustrates the molecular behavior of a. pressure and temperature were fairly. Does Water Temperature Affect Pressure.
From blog.ashcroft.com
How Does Temperature Affect Pressure Gauge Performance? Does Water Temperature Affect Pressure early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. The following figure illustrates the molecular behavior of a. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. in this section we will look. Does Water Temperature Affect Pressure.
From docslib.org
Relationship Between Density, Pressure, and Temperature DocsLib Does Water Temperature Affect Pressure pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. Liquid water properties at temperatures between melting point and boiling point. early scientists. Does Water Temperature Affect Pressure.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does Water Temperature Affect Pressure early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. yes, at constant density, the pressure increases as the temperature does: pressure and temperature were. Does Water Temperature Affect Pressure.
From sciencenotes.org
How to Boil Water at Room Temperature Does Water Temperature Affect Pressure pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. yes, at constant density, the pressure increases as the temperature does: Liquid water properties at temperatures between melting point and boiling point. lets say we have a tank with a fixed mass. Does Water Temperature Affect Pressure.
From chem.libretexts.org
13.4 Effects of Temperature and Pressure on Solubility Chemistry Does Water Temperature Affect Pressure in this section we will look at how the pressure and temperature effect solubility. pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. yes, at constant density, the pressure increases as the temperature does: the change in specific volume for. Does Water Temperature Affect Pressure.
From www.michiganseagrant.org
Properties of Water Teaching Great Lakes Science Does Water Temperature Affect Pressure When we look at pressure effects, we are considering the. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. Liquid water properties at temperatures between melting point and boiling point. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v),. Does Water Temperature Affect Pressure.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Does Water Temperature Affect Pressure yes, at constant density, the pressure increases as the temperature does: Liquid water properties at temperatures between melting point and boiling point. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. pressure and temperature were fairly well understood in the age of newton. Does Water Temperature Affect Pressure.
From www.researchgate.net
Pressuretemperature phase diagram of water [16]. Download High Does Water Temperature Affect Pressure in this section we will look at how the pressure and temperature effect solubility. Liquid water properties at temperatures between melting point and boiling point. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. When we look at pressure effects, we are considering the.. Does Water Temperature Affect Pressure.
From www.boiler-planning.com
Boiling pressure and temperature Bosch Steam boiler planning Does Water Temperature Affect Pressure pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. yes, at constant density, the pressure increases as the temperature does: the change in specific volume for a given change in temperature is not the same at various beginning temperatures. Liquid water. Does Water Temperature Affect Pressure.
From www.h2xengineering.com
Correlation Between Water Temperature and Friction Loss in Pipes Does Water Temperature Affect Pressure Liquid water properties at temperatures between melting point and boiling point. pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. in this section we will look at how the pressure and temperature effect solubility. early scientists explored the relationships among the. Does Water Temperature Affect Pressure.
From atlas-scientific.com
How Does Temperature Affect Dissolved Oxygen? Atlas Scientific Does Water Temperature Affect Pressure pressure and temperature were fairly well understood in the age of newton and galileo, hundreds of years before there was any firm evidence that atoms. When we look at pressure effects, we are considering the. The following figure illustrates the molecular behavior of a. yes, at constant density, the pressure increases as the temperature does: Liquid water properties. Does Water Temperature Affect Pressure.
From studylib.net
How Does Temperature Affect Viscosity, Density and Buoyancy? Does Water Temperature Affect Pressure the change in specific volume for a given change in temperature is not the same at various beginning temperatures. in this section we will look at how the pressure and temperature effect solubility. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding. . Does Water Temperature Affect Pressure.
From www.slideserve.com
PPT Warmup PowerPoint Presentation, free download ID230026 Does Water Temperature Affect Pressure lets say we have a tank with a fixed mass of liquid at atmospheric pressure and room temperature. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. yes, at constant density, the pressure increases as the temperature does: Liquid water properties at temperatures between melting point and. Does Water Temperature Affect Pressure.