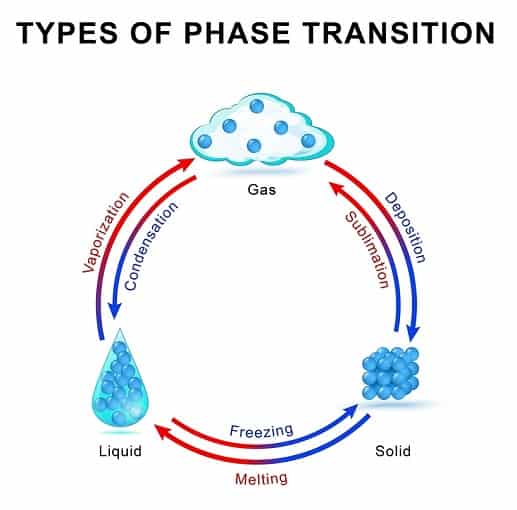
Phase Diagram Sublimation . A phase diagram is a plot of pressure versus temperature for a substance. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. Solid, liquid, gas, and a supercritical fluid. Sublimation is the term for a phase change that proceeds directly from the solid phase to the vapor phase without first making the transition to the. As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. These diagrams indicate the physical states that exist under. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. To be able to identify the triple point, the critical point, and four regions:
from mungfali.com
As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. Solid, liquid, gas, and a supercritical fluid. To be able to identify the triple point, the critical point, and four regions: Sublimation is the term for a phase change that proceeds directly from the solid phase to the vapor phase without first making the transition to the. Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. These diagrams indicate the physical states that exist under. A phase diagram is a plot of pressure versus temperature for a substance.
Phase Diagram Sublimation
Phase Diagram Sublimation Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. A phase diagram is a plot of pressure versus temperature for a substance. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. These diagrams indicate the physical states that exist under. To be able to identify the triple point, the critical point, and four regions: [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. Solid, liquid, gas, and a supercritical fluid. Sublimation is the term for a phase change that proceeds directly from the solid phase to the vapor phase without first making the transition to the.
From animalia-life.club
Sublimation Phase Diagram Phase Diagram Sublimation [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. A phase diagram is a plot of. Phase Diagram Sublimation.
From animalia-life.club
Sublimation Phase Diagram Phase Diagram Sublimation As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. A phase diagram is a plot of pressure versus temperature for a substance. To be able to identify the triple point, the critical point, and four regions: Solid, liquid,. Phase Diagram Sublimation.
From mungfali.com
Phase Diagram Sublimation Phase Diagram Sublimation Solid, liquid, gas, and a supercritical fluid. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. These diagrams indicate the physical states that exist under. Sublimation is the process by which a substance transitions directly from the solid. Phase Diagram Sublimation.
From www.researchgate.net
Schematization of a typical sublimation cycle showing the different Phase Diagram Sublimation Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. Sublimation is the term for a phase change that proceeds directly from the solid phase to the vapor phase without first making the transition to the. These diagrams indicate. Phase Diagram Sublimation.
From sciencetrends.com
What Is Sublimation In Chemistry? Science Trends Phase Diagram Sublimation These diagrams indicate the physical states that exist under. As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. Sublimation is the term for a phase change that proceeds directly from the. Phase Diagram Sublimation.
From animalia-life.club
Sublimation Phase Diagram Phase Diagram Sublimation A phase diagram is a plot of pressure versus temperature for a substance. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. These diagrams indicate the physical states that exist under. To be able to identify the triple point, the critical point, and four regions: Solid, liquid, gas, and a supercritical fluid. Sublimation is. Phase Diagram Sublimation.
From marspedia.org
Deprecated Phase Diagram Sublimation As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. To be able to identify the triple point, the critical point, and four regions: A phase diagram is a plot of pressure versus temperature for a substance. These diagrams indicate the physical states that exist under. Sublimation is an endothermic process that happens below. Phase Diagram Sublimation.
From van.physics.illinois.edu
Q & A Sublimation and Phases. Department of Physics University of Phase Diagram Sublimation A phase diagram is a plot of pressure versus temperature for a substance. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. Sublimation is the term for a phase change that proceeds directly from the solid phase to the vapor phase without first making the transition to the. To be able to identify. Phase Diagram Sublimation.
From animalia-life.club
Sublimation Phase Diagram Phase Diagram Sublimation [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. Solid, liquid, gas, and a supercritical fluid. Sublimation is the term for a phase change that proceeds directly from the solid phase to the vapor phase without first making the transition to the. A phase diagram is a plot of pressure versus temperature for a. Phase Diagram Sublimation.
From animalia-life.club
Sublimation Phase Diagram Phase Diagram Sublimation Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. These diagrams indicate the physical states that exist under. Sublimation is an endothermic process that happens below a substance's triple point in. Phase Diagram Sublimation.
From saylordotorg.github.io
Phase Diagrams Phase Diagram Sublimation As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. These diagrams indicate the physical states that exist under. Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. Solid, liquid, gas, and a supercritical fluid. Sublimation is an endothermic process that. Phase Diagram Sublimation.
From mungfali.com
Phase Diagram Sublimation Phase Diagram Sublimation Solid, liquid, gas, and a supercritical fluid. A phase diagram is a plot of pressure versus temperature for a substance. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. To be able to identify the triple point, the critical point, and four regions: Sublimation is an endothermic process that happens below a substance's triple. Phase Diagram Sublimation.
From studiousguy.com
7 Sublimation Examples in Daily Life StudiousGuy Phase Diagram Sublimation Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. These diagrams indicate the physical states that exist under. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. Sublimation is the term for a phase change that proceeds directly from the. Phase Diagram Sublimation.
From physics.stackexchange.com
thermodynamics phase transition by sublimation Physics Stack Exchange Phase Diagram Sublimation Solid, liquid, gas, and a supercritical fluid. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. These diagrams indicate the physical states that exist under. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. A phase diagram is a plot of pressure versus temperature for a substance.. Phase Diagram Sublimation.
From mungfali.com
Phase Diagram Sublimation Phase Diagram Sublimation Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. To be able to identify the triple point, the critical point, and four regions: A phase diagram is a plot of pressure versus temperature for a substance. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. As with. Phase Diagram Sublimation.
From animalia-life.club
Sublimation Phase Diagram Phase Diagram Sublimation A phase diagram is a plot of pressure versus temperature for a substance. Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. Solid, liquid, gas, and a supercritical fluid. As with evaporation,. Phase Diagram Sublimation.
From mungfali.com
Phase Diagram Sublimation Phase Diagram Sublimation To be able to identify the triple point, the critical point, and four regions: Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. Sublimation is the term for a phase change that proceeds directly from the solid phase to the vapor phase without first making the transition. Phase Diagram Sublimation.
From animalia-life.club
Sublimation Phase Diagram Phase Diagram Sublimation Solid, liquid, gas, and a supercritical fluid. To be able to identify the triple point, the critical point, and four regions: These diagrams indicate the physical states that exist under. A phase diagram is a plot of pressure versus temperature for a substance. As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. Sublimation. Phase Diagram Sublimation.
From mungfali.com
Phase Diagram Sublimation Phase Diagram Sublimation Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. These diagrams indicate the physical states that exist under. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. To be able to identify the triple point, the critical point, and four regions:. Phase Diagram Sublimation.
From ar.inspiredpencil.com
Sublimation Phase Diagram Phase Diagram Sublimation [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. A phase diagram is a plot of pressure versus temperature for a substance. Sublimation is the process by which a substance transitions directly from the solid phase to the. Phase Diagram Sublimation.
From animalia-life.club
Sublimation Phase Diagram Phase Diagram Sublimation Solid, liquid, gas, and a supercritical fluid. These diagrams indicate the physical states that exist under. Sublimation is the term for a phase change that proceeds directly from the solid phase to the vapor phase without first making the transition to the. To be able to identify the triple point, the critical point, and four regions: [3] figure 2 shows. Phase Diagram Sublimation.
From animalia-life.club
Sublimation Phase Diagram Phase Diagram Sublimation As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. Solid, liquid, gas, and a supercritical fluid. To be able to identify the triple point, the critical point, and four regions: Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. [3] figure 2 shows a phase diagram. Phase Diagram Sublimation.
From phasediagrams.weebly.com
Types of Phase Diagrams Phase Diagrams Phase Diagram Sublimation These diagrams indicate the physical states that exist under. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. To be able to identify the triple point, the critical point, and four regions: Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid. Phase Diagram Sublimation.
From unistudium.unipg.it
Phase Diagrams Phase Diagram Sublimation As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. To be able to identify the triple point, the critical point, and four regions: A phase diagram is a plot of pressure versus temperature for a substance. Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous. Phase Diagram Sublimation.
From pharmaperception.blogspot.com
Pharmaperception Sublimation science of Primary Drying in Phase Diagram Sublimation Solid, liquid, gas, and a supercritical fluid. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. To be able to identify the triple point, the critical point, and four regions: Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. A phase diagram is a plot of pressure. Phase Diagram Sublimation.
From atocwatervapor.blogspot.com
Water Phase Change Sublimation Phase Diagram Sublimation A phase diagram is a plot of pressure versus temperature for a substance. Solid, liquid, gas, and a supercritical fluid. These diagrams indicate the physical states that exist under. Sublimation is the term for a phase change that proceeds directly from the solid phase to the vapor phase without first making the transition to the. Sublimation is the process by. Phase Diagram Sublimation.
From www.expii.com
Sublimation — Definition & Overview Expii Phase Diagram Sublimation These diagrams indicate the physical states that exist under. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the. Phase Diagram Sublimation.
From narodnatribuna.info
Sublimation Phase Change Britannica Phase Diagram Sublimation Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. These diagrams indicate the physical states that exist under. To be able to identify the triple point, the critical point, and four. Phase Diagram Sublimation.
From animalia-life.club
Sublimation Phase Diagram Phase Diagram Sublimation Solid, liquid, gas, and a supercritical fluid. Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. These diagrams indicate the physical states that exist under. To be able to identify the triple. Phase Diagram Sublimation.
From animalia-life.club
Sublimation Phase Diagram Phase Diagram Sublimation Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. These diagrams indicate the physical states that. Phase Diagram Sublimation.
From www.slideserve.com
PPT Phase Diagram PowerPoint Presentation, free download ID2997497 Phase Diagram Sublimation Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. A phase diagram is a plot of pressure versus temperature for a substance. As with evaporation, sublimation is possible within the whole range of temperatures t and pressures p. [3] figure 2 shows a phase diagram for carbon. Phase Diagram Sublimation.
From mungfali.com
Phase Diagram Sublimation Phase Diagram Sublimation [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. To be able to identify the triple point, the critical point, and four regions: Sublimation is the process by which a substance transitions directly from the solid phase to. Phase Diagram Sublimation.
From ar.inspiredpencil.com
Sublimation Phase Diagram Phase Diagram Sublimation Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. To be able to identify the triple point, the critical point, and four regions: Solid, liquid, gas, and a supercritical fluid. Sublimation is the term for a phase change that proceeds directly from the solid phase to the vapor phase without first making the. Phase Diagram Sublimation.
From www.researchgate.net
43 Simplified water phase diagram. The blue arrow depicts the Phase Diagram Sublimation To be able to identify the triple point, the critical point, and four regions: [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. A phase diagram is a plot of pressure versus temperature for a substance. Sublimation is an endothermic process that happens below a substance's triple point in its phase diagram. Solid, liquid,. Phase Diagram Sublimation.
From animalia-life.club
Sublimation Phase Diagram Phase Diagram Sublimation [3] figure 2 shows a phase diagram for carbon dioxide where sublimation would occur below. Sublimation is the term for a phase change that proceeds directly from the solid phase to the vapor phase without first making the transition to the. These diagrams indicate the physical states that exist under. To be able to identify the triple point, the critical. Phase Diagram Sublimation.